SCD stem cell–derived sensory neurons (iSNs) exhibit pronounced sensitization to electrical stimulation.
SCD plasma sensitizes SCD iSNs to transient receptor potential cation channel subfamily V member 1 stimulation by capsaicin.
Visual Abstract
Individuals living with sickle cell disease (SCD) experience severe recurrent acute and chronic pain. Challenges to gaining mechanistic insight into pathogenic SCD pain processes include differential gene expression and function of sensory neurons between humans and mice with SCD, and extremely limited availability of neuronal tissues from patients with SCD. Here, we used induced pluripotent stem cells (iPSCs), derived from patients with SCD, differentiated into sensory neurons (SCD iSNs) to begin to overcome these challenges. We characterize key gene expression and function of SCD iSNs to establish a model to investigate intrinsic and extrinsic factors that may contribute to SCD pain. Despite similarities in receptor gene expression, SCD iSNs show pronounced excitability using patch clamp electrophysiology. Furthermore, we find that plasma taken from patients with SCD during acute pain associated with a vaso-occlusive event increases the calcium responses to the nociceptive stimulus capsaicin in SCD iSNs compared with those treated with paired plasma from patients with SCD at steady state baseline or healthy control plasma samples. We identified high levels of the polyamine spermine in baseline and acute pain states of plasma from patients with SCD, which sensitizes SCD iSNs to subthreshold concentrations of capsaicin. Together, these data identify potential intrinsic mechanisms within SCD iSNs that may extend beyond a blood-based pathology.
Introduction
Sickle cell disease (SCD), the most common inherited hemoglobinopathy, affects >100 000 Black and Hispanic Americans,1-3 and >3 million individuals globally.1-3 SCD pathophysiology includes red blood cell sickling, chronic hemolysis, vaso-occlusion with resultant ischemia-reperfusion injury, and chronic vascular inflammation, leading to multiorgan damage and premature death. Individuals with SCD often suffer from both acute4-8 and chronic (steady state) pain9-13 that cannot be explained by known SCD pathology.13-16 Opioids are the main analgesic for acute and chronic SCD pain; however, they are fraught with serious aversive side effects16-19 and often do not provide effective analgesia. SCD disproportionately affects Black individuals who also experience multifactorial health care disparities including reduced likelihood for pain treatment.20-22 These disparities, the current opioid crisis, and ineffective analgesia of opioids, create a critical need for effective nonopioid SCD pain therapies. To accomplish this goal, improved understanding of the neurobiological mechanisms underlying human SCD pain is needed.
Humanized SCD mouse models exhibit pronounced acute and chronic pain behaviors.23-27 However, growing evidence indicates striking differences in gene expression, transcriptional regulators, and functional responsiveness between human and mouse dorsal root ganglia (DRG) sensory neurons.28-36 Human DRGs may constitute a translational bridge between preclinical rodent models and clinical testing of targets.33,36-40 However, obtaining DRGs from individuals with SCD is highly impractical because of rare clinical indications for DRG resection.
Human induced pluripotent stem cell (iPSC)-derived sensory neurons (iSNs) have recently been used to model chronic pain disorders.41 iSNs express canonical markers of human nociceptors42-51 and exhibit functional responses to noxious stimuli.47,49,51 Therefore, we differentiated iSNs from individuals with SCD and age- and race-matched healthy controls (HCs) and observed sensitization to electrical simulation and SCD plasma and plasma-derived factors in SCD iSNs compared with HC iSNs. Importantly, this study establishes a human-specific model of SCD and proposes the novel finding that both intrinsic and extrinsic factors may underly SCD pain.
Materials and methods
iPSC and iSN differentiation
We used 3 SCD lines and 3 age- and race-matched HC iPSC lines (WiCell, Madison, WI; supplemental Table 1, available on Blood website). SCD donors were physician diagnosed with hemoglobin-β (Hb-β [HBB]) HBB(E6V) sickle cell anemia (HbSS genotype). Karyotypically normal and mycoplasma-negative iPSCs were maintained on Matrigel (Corning) or Geltrex (Gibco) in Essential 8 medium (Gibco) and passaged every 4 to 6 days. iPSCs were differentiated into iSNs based on previous reports.41,49 After dual SMAD inhibition (100 nM LDN 193189, 10 μM SB 431542, Selleck Chemical), SNs were patterned using 3 μM CHIR99021, 10 μM SU5402, and 10 μM DAPT (Selleck Chemical) starting on day 2 with a stepwise addition of N2 medium (25% Dulbecco modified Eagle medium, 25% F12, 50% neurobasal medium, 1× N2 supplement, 1× B27 supplement; ThermoFisher) every other day starting on day 4. On day 12, cells were dissociated using Accutase (ThermoFisher) and replated onto triple-coated (15 μg/mL poly-L-ornithine hydrobromide [Sigma], 2 μg/mL laminin [Fisher Scientific], and 2 μg/mL fibronectin [Fisher Scientific]) 96-well plates (20 000 cells per well), 6-well plates (480 000 cells per well), or glass coverslips (52 500 cells per coverslip) in 24-well plates and maintained in N2 media supplemented with neuronal growth factors (10 ng/mL human nerve growth factor β, neurotrophin-3, brain-derived neurotrophic factor, and Glial cell line–derived neurotrophic factor; PeproTech) throughout the duration of the experiments. iSNs were considered mature at day 35 and used experimentally between days 35 to 45 (supplemental Figure 1).
Immunocytochemistry
Cells were fixed in 4% paraformaldehyde for 20 minutes at room temperature and rinsed with phosphate-buffered saline (PBS). After blocking and permeabilization, cells were incubated with primary antibodies overnight at 4°C, then labeled with appropriate secondary antibodies (supplemental Table 2). Nuclei were labeled with DAPI (4′,6-diamidino-2-phenylindole). Representative images were taken on a Zeiss confocal microscope using a 20× air objective and are displayed as a maximum intensity projection of z-stack image series for Figure 1 and supplemental Figures 1 and 2.
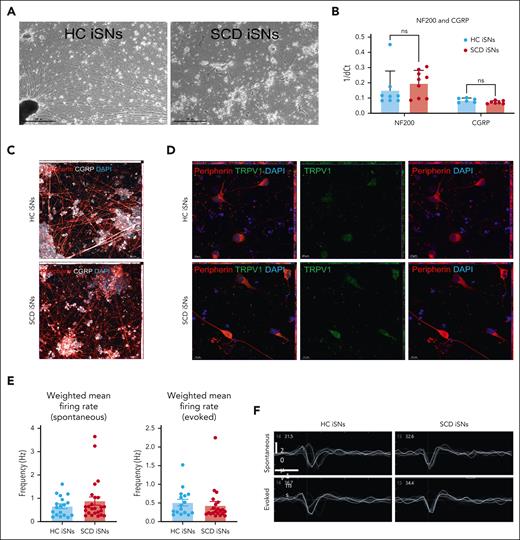
SCD iPSCs and age- and race-matched iPSCs HCs differentiated into sensory neurons (iSNs) express canonical markers and exhibit function indicative of mature human nociceptors. HC and SCD iSNs: brightfield images at original magnification ×4 (scale bar, 500 μm) express transcripts specific for mature sensory neurons (A); NF200 and calcitonin gene-related peptide (CGRP) as analyzed via qRT-PCR (B). Immunocytochemistry (ICC) shows robust protein expression of peripherin (red), CGRP (white), and TRPV1 (green) in both HC and SCD iSNs at original magnification ×20 (scale bar, 50 μm) (C) and original magnification ×63 (scale bar, 10 μm) (D). Images are maximum intensity projections (MIPs) from z-stack series taken on a Zeiss confocal microscope. (E) Spontaneous (left) and electrically evoked (right) weighted mean firing data (frequency; Hz) from HC and SCD iSNs; n = 2 independent differentiations. (F) Representative extracellular recorded waveforms of HC and SCD iSNs during spontaneous and electrically evoked recordings.
SCD iPSCs and age- and race-matched iPSCs HCs differentiated into sensory neurons (iSNs) express canonical markers and exhibit function indicative of mature human nociceptors. HC and SCD iSNs: brightfield images at original magnification ×4 (scale bar, 500 μm) express transcripts specific for mature sensory neurons (A); NF200 and calcitonin gene-related peptide (CGRP) as analyzed via qRT-PCR (B). Immunocytochemistry (ICC) shows robust protein expression of peripherin (red), CGRP (white), and TRPV1 (green) in both HC and SCD iSNs at original magnification ×20 (scale bar, 50 μm) (C) and original magnification ×63 (scale bar, 10 μm) (D). Images are maximum intensity projections (MIPs) from z-stack series taken on a Zeiss confocal microscope. (E) Spontaneous (left) and electrically evoked (right) weighted mean firing data (frequency; Hz) from HC and SCD iSNs; n = 2 independent differentiations. (F) Representative extracellular recorded waveforms of HC and SCD iSNs during spontaneous and electrically evoked recordings.
qRT-PCR
RNA was isolated from HC and SCD iSNs using the RNeasy Mini Kit (Qiagen) following the manufacturer’s instructions, quantified using a Nanodrop spectrophotometer, treated with RQ1 RNase-free DNase (Promega), and converted to complementary DNA using the Promega Reverse Transcription system (Promega). SYBR green quantitative reverse transcription polymerase chain reaction (qRT-PCR) was performed in triplicate using complementary DNA and run on the Bio-Rad CFX384 thermocycler. Primer sequences are shown in supplemental Table 3. Cq values for each target were normalized to glyceraldehyde-3-phosphate dehydrogenase and calculated using the ΔΔCq method. A minimum of 3 differentiations for each line were collected and run in technical triplicates.
MEA
On day 12 of differentiation, cells were dissociated and plated onto PLL (poly-L-lysine)-laminin-fibronectin–coated 48-well CytoView microelectrode array plates (Axion Biosystems) at a density of 30 000 cells per well. Spontaneous and electrically evoked motor neuron activity was recorded every other day for 2 weeks starting from day 16 using the first-generation Maestro system (Axion Biosystems). Cells were maintained on a preheated (37°C) multielectrode array (MEA) stage for 10 minutes before starting the 4-minute recordings. Version 2.4.2.13 of the AxIS acquisition software (Axion Biosystems) was used to record activity across 16 electrodes per well with a sampling frequency of 12.5 kHz and a digital high-pass filter of 5 Hz IIR (infinite impulse response). The spike-detecting threshold of 5 spikes per minute was defined as voltage exceeding 6 standard deviations away from the mean background noise. Butterworth digital filter settings with high (200 Hz) and low (3 kHz) pass cutoff frequencies were applied during recordings. For electrically evoked recordings, the neural stimulation with artifact elimination (0.5 V for 400 μs [36 400 μs total operation time]) was applied to the cells by stimulating 1 electrode per well once every 10 seconds. Statistic Compiler files and waveform images were extracted from raw AxIS (spontaneous recordings) and raw AxIS Artifact Eliminator files (electrically evoked recordings) for weighted mean firing rates. Values <0.15 Hz were considered background noise, as previously determined52 and were removed from data sets. Analyses were performed using PENN022i-89-1 and CREM032i-SS48.
Calcium imaging and flux assay
Coverslips were incubated in 2.5 μg/mL of the ratiometric dye Fura-2-AM in 2% bovine serum albumin for 45 minutes, followed by a 30-minute wash in extracellular normal HEPES (N-2-hydroxyethylpiperazine-N’-2-ethanesulfonic acid) buffer (ENH) before imaging. Coverslips were mounted on a Nikon Eclipse TE200 inverted microscope and were superfused with ENH (pH 7.4 ± 0.03; 320 ± 3 mOsm; 150 mM NaCl, 10 mM HEPES, 8 mM glucose, 5.6 mM KCl, 2 mM CaCl2, and 1 mM MgCl2; Sigma Aldrich). Fluorescence images were obtained at 340 and 380 nm using Nikon Elements software (Nikon Instruments). Following a 1-minute baseline incubation in ENH, neurons were exposed to 100 μM glutamate (abcam) or 50 μM α,β-methyleneadenosine 5’-triphosphate trisodium salt (ab-meATP; Tocris) for 2 minutes; or 0.1 μM, 1 μM, 10 μM, or 50 μM capsaicin (Sigma Aldrich) for 30 seconds, ENH for 3 minutes, and 50 mM KCl (Sigma Aldrich) for 1 minute. All buffers were superfused at a rate of 6 mL/min. Neurons displaying a ≥20% increase in the 340:380 nm ratio relative to baseline in response to agonist treatment were included in the quantification.
Calcium flux was measured in iSNs seeded in a 96-well plate using the Fluo-4 NW Calcium Assay Kit (ThermoFisher) per the manufacturer’s instructions. Growth medium was removed, and 100 μL of dye loading solution was added to each well and incubated for 30 minutes at 37°C followed by 30 minutes at room temperature. Just before measuring fluorescence, 25 μL of the appropriate agonist or PBS were spiked into each well. Fluorescence (excitation, 494 nm; emission, 516 nm) was immediately measured using a GloMax microplate reader. No differences in fluorescence were found between preagonist background fluorescence and PBS-stimulated wells (supplemental Figure 3); relative fluorescence (% above PBS baseline) was calculated by subtracting the fluorescence of PBS-stimulated wells from each test well, then dividing by PBS-stimulated well value, and multiplying by 100. Agonist solutions were made fresh daily and included 250 mM KCl (final concentration, 50 mM); 500 μM L-glutamate (final concentration, 100 μM); 250 μM ab-meATP (final concentration, 50 μM); and 250 μM, 50 μM, 5 μM, or 0.5 μM capsaicin (final concentration, 50 μM, 10 μM, 1 μM, or 0.1 μM).
Human plasma treatments and metabolomics
Human samples were collected under an institutional human research review board approved protocol. Written informed consent was obtained from the study participant and/or legal guardian, and written informed assent was obtained from the participant when age appropriate.
Human plasma samples, collected via routine venipuncture, were obtained from individuals with SCD during baseline health (SS BL) and during hospitalization for an acute pain event (SS IP) as paired samples from the same individual, and from healthy Black individuals without SCD (HCs; supplemental Table 4). Plasma was processed and immediately stored at −80°C. Plasma was thawed overnight at 4°C on ice before usage; freeze/thaw cycles were limited to 2. iSNs were treated with 10% plasma diluted in iSN maturation media. After a 30-minute treatment, all media were removed, and 100 μL of dye loading solution was immediately added for the Fluo-4 NW calcium flux assay.
SS BL with paired SS IP samples and race-matched and relatively age-matched HC plasma samples (supplemental Table 5; n = 25 per group) were analyzed by Metabolon, Inc, using its in-house methods for sample processing, ultrahigh performance liquid chromatography–tandem mass spectroscopy, quantification and data normalization, and statistical analyses (Welch 2-sample t tests and matched-pairs t tests).
iSN sensitization and ETR blocking treatments
iSNs were treated with 100 ng/mL recombinant human endothelin 1 (ET1; R&D) or C-C motif chemokine ligand 2 (CCL2; PeproTech) diluted in iSN maturation media. After a 30-minute treatment, cells were immediately processed for the Fluo-4 NW calcium flux assay. Recombinant human protein treatments (ET1 and CCL2) were performed on only PENN022i-89-1 and CREM032i-SS48-1 lines in biological triplicates by incubating iSNs with 100 μM endothelin receptor type A (ETRa) antagonist BQ123 (Tocris) or 100 μM ETRb antagonist BQ788 (Tocris) diluted in iSN maturation media for 24 hours at 37°C before 30-minute ET1 or plasma treatment. Finally, 30-minute spermine treatments were performed by treating iSNs with 100 nM, 100 μM, or 500 μM spermine (Sigma) diluted in iSN maturation media for 30 minutes at 37°C before the Fluo-4 NW calcium flux assay.
Whole-cell patch clamp electrophysiology
Whole-cell patch clamp recordings in current-clamp mode were performed on the PENN022i-89-1 and CREM032i-SS48 iSNs. iSNs were continuously superfused with extracellular solution: 140 mM NaCl, 2.8 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, 10 mM glucose, and 8.8 mM sucrose (pH 7.4 and 310 mOsm). Patch electrodes were made from borosilicate glass capillaries with 4-8MΩ resistance when filled with internal solution: 135 mM KCl, 4.1 mM MgCl2, 2 mM EGTA, 0.2 mM NaGTP, 2.5 mM ATPNa2, and 10 mM HEPES (pH 7.2 and 290 mOsm). Recordings we performed at room temperature using either EPC10 amplifier with Patchmaster software (HEKA Electronics) or Axopatch 200B amplifier with pCLAMP software (Molecular Devices). Cells were held at −60 mV, and neuronal excitability was examined using a series of 500-ms depolarizing current pulses (range, 0-190 pA; 10-pA increments).
Statistical analyses
Experiments were performed in technical triplicates for a minimum of 3 independent experiments unless otherwise noted. Data were analyzed using GraphPad Prism software, and the appropriate statistical tests were performed including regression and outlier removal (ROUT) analysis to remove statistical outliers, Student t test, Welch t test, paired 2-sample t test, 1-way analysis of variance (ANOVA), and 2-way ANOVA followed by Tukey post hoc analysis of significance. Changes were considered statistically significant when P < .05.
Results
HC and SCD iPSC-derived SNs express canonical markers of mature and functional human sensory neurons
Three SCD iPSC lines and 3 age- and race-matched HC iPSC lines were differentiated into iSNs following published protocols41,49 (Figure 1A; supplemental Figures 1 and 2). HC and SCD iSNs showed similar transcript expression levels for the mature neuron marker neurofilament heavy chain (NF200) and the sensory neuron marker calcitonin gene-related peptide as measured by qRT-PCR (Figure 1B). Immunocytochemistry confirmed appropriate neuronal morphology and expression of calcitonin gene-related peptide (CGRP), type III intermediate filament protein (peripherin), and transient receptor potential cation channel subfamily V member 1 (TRPV1; Figure 1C-D) with virtually no expression of the glial marker glial acidic fibrillary protein or proliferative marker Ki67 (supplemental Figures 1 and 2). MEA recordings detected spontaneous and electrically evoked action potentials with no differences in weighted mean firing rate between HC and SCD iSNs (Figure 1E-F) indicating iSN maturation.
Next, we used qRT-PCR to assess expression of sensory neuron and pain-relevant ligands and receptors (Table 1) in healthy and disease iSN (Table 2). We found no significant differences in cytokine receptor transcripts, including CCL2, despite their involvement in peripheral immune response and inflammatory pain,53-57 or in receptors specific for peripheral immune response and vasoconstriction including ETRa and ETRb (Table 2). Similarly, we found no difference in expression levels between HC and SCD iSNs for nociceptor-relevant ion channels (Table 2) and ion channels potentially involved in sensitization of sensory neurons during injury (Table 2). These findings indicate that SCD iPSCs and the age- and race-matched HCs can be consistently differentiated into iSNs expressing canonical and functional sensory neuron markers.
Key sensory neuron gene qRT-PCR targets and any known associations with SCD
| Target . | Protein . | Function . | Association with SCD . | Reference . |
|---|---|---|---|---|
| CACNA2D1 | Calcium channel subunit | Voltage-gated calcium channel | Potentially involved in spontaneous pain in SCD | 96 |
| CCL2 | Cytokine | Recruitment of immune cells | Upregulated during SCD | 55,97 |
| CCR1 | Chemokine receptor | Recruitment of immune cells | Differentially expressed in SCD | 56 |
| CCR2 | Chemokine receptor | Recruitment of immune cells | Contributes to hypersensitivity in SCD | 53 |
| CCR3 | Chemokine receptor | Recruitment of immune cells | None reported | — |
| CCR5 | Chemokine receptor | Recruitment of immune cells | Immune/inflammatory response in SCD | 57 |
| CD4 | Glycoprotein | T-cell co-receptor | Decreased in SCD | 98 |
| CGRP | Neuropeptide | Vasodilator | Upregulated during SCD | 23,99,100 |
| CHRNA9 | Ion channel subunit | Ligand-gated ion channel | None reported | — |
| CRLF2 | Cytokine receptor | Cell proliferation | None reported | — |
| CSF2Ra | Cytokine receptor subunit | Production of hematopoietic cells | None reported | — |
| CSF2Rb | Cytokine receptor subunit | Production of hematopoietic cells | Hydroxyurea metabolism | 101 |
| ETRa | Endothelin receptor | Vasoconstriction | Elevated in SCD; Contributes to hypersensitivity | 64,102 |
| ETRb | Endothelin receptor | Vasoconstriction | Red blood cell dehydration in SCD | 103 |
| HCN1 | Membrane channel | Hyperpolarization-activated cation channel | None reported | — |
| Nav1.7 | Sodium channel | Voltage-gated sodium channel | Associated with pain pathways | 104 |
| NF200 | Intermediate filament | Neuronal cytoskeleton | Associated with sensory neurons | 105 |
| P2X3 | Ion channel | Ligand-gated ion channel | Associated with sensory neurons | 105 |
| P2X4 | Ion channel | Ligand-gated ion channel | Speculated to be involved in red blood cell activity | 106 |
| Piezo1 | Ion channel | Mechanosensitive ion channel | Red blood cell dehydration in SCD | 107 |
| Piezo2 | Ion channel | Mechanosensitive ion channel | None reported | — |
| TRPA1 | Ion channel | Nonselective cation channel | Associated with pain in SCD | 108 |
| TRPC5 | Ion channel | Nonselective cation channel | Contributes to hypersensitivity in SCD | 105 |
| TRPM3 | Ion channel | Nonselective cation channel | None reported | — |
| TRPM8 | Ion channel | Nonselective cation channel | Speculated to be involved in hypersensitivity in SCD | 27 |
| TRPV1 | Calcium channel | Nonselective cation channel | Contributes to hypersensitivity in SCD | 24 |
| TRPV4 | Calcium channel | Nonselective cation channel | Contributes to hypersensitivity in SCD | 109 |
| Target . | Protein . | Function . | Association with SCD . | Reference . |
|---|---|---|---|---|
| CACNA2D1 | Calcium channel subunit | Voltage-gated calcium channel | Potentially involved in spontaneous pain in SCD | 96 |
| CCL2 | Cytokine | Recruitment of immune cells | Upregulated during SCD | 55,97 |
| CCR1 | Chemokine receptor | Recruitment of immune cells | Differentially expressed in SCD | 56 |
| CCR2 | Chemokine receptor | Recruitment of immune cells | Contributes to hypersensitivity in SCD | 53 |
| CCR3 | Chemokine receptor | Recruitment of immune cells | None reported | — |
| CCR5 | Chemokine receptor | Recruitment of immune cells | Immune/inflammatory response in SCD | 57 |
| CD4 | Glycoprotein | T-cell co-receptor | Decreased in SCD | 98 |
| CGRP | Neuropeptide | Vasodilator | Upregulated during SCD | 23,99,100 |
| CHRNA9 | Ion channel subunit | Ligand-gated ion channel | None reported | — |
| CRLF2 | Cytokine receptor | Cell proliferation | None reported | — |
| CSF2Ra | Cytokine receptor subunit | Production of hematopoietic cells | None reported | — |
| CSF2Rb | Cytokine receptor subunit | Production of hematopoietic cells | Hydroxyurea metabolism | 101 |
| ETRa | Endothelin receptor | Vasoconstriction | Elevated in SCD; Contributes to hypersensitivity | 64,102 |
| ETRb | Endothelin receptor | Vasoconstriction | Red blood cell dehydration in SCD | 103 |
| HCN1 | Membrane channel | Hyperpolarization-activated cation channel | None reported | — |
| Nav1.7 | Sodium channel | Voltage-gated sodium channel | Associated with pain pathways | 104 |
| NF200 | Intermediate filament | Neuronal cytoskeleton | Associated with sensory neurons | 105 |
| P2X3 | Ion channel | Ligand-gated ion channel | Associated with sensory neurons | 105 |
| P2X4 | Ion channel | Ligand-gated ion channel | Speculated to be involved in red blood cell activity | 106 |
| Piezo1 | Ion channel | Mechanosensitive ion channel | Red blood cell dehydration in SCD | 107 |
| Piezo2 | Ion channel | Mechanosensitive ion channel | None reported | — |
| TRPA1 | Ion channel | Nonselective cation channel | Associated with pain in SCD | 108 |
| TRPC5 | Ion channel | Nonselective cation channel | Contributes to hypersensitivity in SCD | 105 |
| TRPM3 | Ion channel | Nonselective cation channel | None reported | — |
| TRPM8 | Ion channel | Nonselective cation channel | Speculated to be involved in hypersensitivity in SCD | 27 |
| TRPV1 | Calcium channel | Nonselective cation channel | Contributes to hypersensitivity in SCD | 24 |
| TRPV4 | Calcium channel | Nonselective cation channel | Contributes to hypersensitivity in SCD | 109 |
Key sensory neuron gene transcript expression analyzed by qRT-PCR for HC and SCD iSNs
| Target . | HC iSN expression (mean ± SE) . | SCD iSN expression (mean ± SE) . | t test (P value) . |
|---|---|---|---|
| Cytokines and receptors | |||
| CCR1 | 1.1303 ± 0.8083 | 1.3154 ± 1.0460 | .8905 |
| CCR2 | 3.1287 ± 3.1055 | 2.2507 ± 1.9219 | .8137 |
| CCR3 | 0.6707 ± 0.5664 | 1.7838 ± 1.7412 | .5573 |
| CCR5 | 1.3152 ± 0.8065 | 0.8818 ± 0.4786 | .6516 |
| CCL2 | 0.0186 ± 0.5340 | 1.0975 ± 0.6934 | .2366 |
| Immune response and vasoconstriction | |||
| CD4 | 0.7275 ± 0.3015 | 1.1605 ± 0.7263 | .5932 |
| CRLF2 | 1.6009 ± 1.1369 | 1.3079 ± 0.7801 | .8347 |
| CSF2Ra | 0.4063 ± 0.1305 | 0.7119 ± 0.5496 | .6019 |
| CSF2Rb | 0.0385 ± 0.2229 | 0.3847 ± 1.2263 | .7878 |
| ETRa | 0.1256 ± 0.6420 | 0.6931 ± 0.6029 | .5285 |
| ETRb | 2.1103 ± 1.7300 | 1.8979 ± 1.5764 | .9288 |
| Ion channels: nociceptor relevant | |||
| HCN1 | 0.7730 ± 0.0928 | 0.7844 ± 0.3887 | .9779 |
| Nav1.7 | 0.6744 ± 0.0729 | 0.5603 ± 0.3913 | .7813 |
| P2X3 | 1.7744 ± 1.4308 | 1.4527 ± 1.0147 | .8570 |
| P2X4 | 0.2109 ± 0.1133 | 0.1079 ± 0.8384 | .9059 |
| CACNA2D1 | 0.3918 ± 0.0937 | 0.0371 ± 0.6868 | .6222 |
| CHRNA9 | 1.8654 ± 1.3584 | 1.6363 ± 1.0630 | .8960 |
| Ion channels: SCD pain specific | |||
| Piezo1 | 0.4244 ± 0.6581 | 0.5183 ± 1.0204 | .9395 |
| Piezo2 | 0.8643 ± 0.6382 | 0.9337 ± 0.7513 | .7360 |
| TRPA1 | 2.7452 ± 2.7365 | 1.6610 ± 1.5499 | .7360 |
| TRPC5 | 2.2992 ± 1.5932 | 2.8119 ± 2.5534 | .8673 |
| TRPM3 | 1.6454 ± 1.1363 | 1.5149 ± 0.9201 | .9301 |
| TRPM8 | 0.0087 ± 0.7276 | 0.0028 ± 0.5776 | .9951 |
| TRPV1 | 0.5636 ± 0.0269 | 0.7488 ± 0.2250 | .4368 |
| TRPV4 | 0.5997 ± 0.1999 | 0.9690 ± 0.5988 | .5718 |
| Target . | HC iSN expression (mean ± SE) . | SCD iSN expression (mean ± SE) . | t test (P value) . |
|---|---|---|---|
| Cytokines and receptors | |||
| CCR1 | 1.1303 ± 0.8083 | 1.3154 ± 1.0460 | .8905 |
| CCR2 | 3.1287 ± 3.1055 | 2.2507 ± 1.9219 | .8137 |
| CCR3 | 0.6707 ± 0.5664 | 1.7838 ± 1.7412 | .5573 |
| CCR5 | 1.3152 ± 0.8065 | 0.8818 ± 0.4786 | .6516 |
| CCL2 | 0.0186 ± 0.5340 | 1.0975 ± 0.6934 | .2366 |
| Immune response and vasoconstriction | |||
| CD4 | 0.7275 ± 0.3015 | 1.1605 ± 0.7263 | .5932 |
| CRLF2 | 1.6009 ± 1.1369 | 1.3079 ± 0.7801 | .8347 |
| CSF2Ra | 0.4063 ± 0.1305 | 0.7119 ± 0.5496 | .6019 |
| CSF2Rb | 0.0385 ± 0.2229 | 0.3847 ± 1.2263 | .7878 |
| ETRa | 0.1256 ± 0.6420 | 0.6931 ± 0.6029 | .5285 |
| ETRb | 2.1103 ± 1.7300 | 1.8979 ± 1.5764 | .9288 |
| Ion channels: nociceptor relevant | |||
| HCN1 | 0.7730 ± 0.0928 | 0.7844 ± 0.3887 | .9779 |
| Nav1.7 | 0.6744 ± 0.0729 | 0.5603 ± 0.3913 | .7813 |
| P2X3 | 1.7744 ± 1.4308 | 1.4527 ± 1.0147 | .8570 |
| P2X4 | 0.2109 ± 0.1133 | 0.1079 ± 0.8384 | .9059 |
| CACNA2D1 | 0.3918 ± 0.0937 | 0.0371 ± 0.6868 | .6222 |
| CHRNA9 | 1.8654 ± 1.3584 | 1.6363 ± 1.0630 | .8960 |
| Ion channels: SCD pain specific | |||
| Piezo1 | 0.4244 ± 0.6581 | 0.5183 ± 1.0204 | .9395 |
| Piezo2 | 0.8643 ± 0.6382 | 0.9337 ± 0.7513 | .7360 |
| TRPA1 | 2.7452 ± 2.7365 | 1.6610 ± 1.5499 | .7360 |
| TRPC5 | 2.2992 ± 1.5932 | 2.8119 ± 2.5534 | .8673 |
| TRPM3 | 1.6454 ± 1.1363 | 1.5149 ± 0.9201 | .9301 |
| TRPM8 | 0.0087 ± 0.7276 | 0.0028 ± 0.5776 | .9951 |
| TRPV1 | 0.5636 ± 0.0269 | 0.7488 ± 0.2250 | .4368 |
| TRPV4 | 0.5997 ± 0.1999 | 0.9690 ± 0.5988 | .5718 |
No significant differences in transcript expression of cytokine receptors, immune response and vasoconstriction related receptors, and ion channels were identified between HC and SCD iSNs as analyzed by qRT-PCR. Values for each target are normalized to housekeeping gene (glyceraldehyde-3-phosphate dehydrogenase) and Cq values for each sample were normalized to a single HC sample value to calculate relative fold change (ΔΔCq) in gene expression. Independent t tests for each target were used to analyze differences in expression between HC and SCD samples.
SE, standard error.
HC and SCD iSNs show similar calcium responses to hyperpolarization
Next, we tested calcium responses in HC and SCD iSNs to 50 mM KCl, 100 μM glutamate, and 50 μM ab-meATP using ratiometric calcium imaging analysis (Figure 2A-B). No differences in percentage of iSNs responding or magnitude of response were found between the HC and SCD iSN (χ2, 1-way ANOVA, P > .05). Previous studies had challenges recording calcium responses to capsaicin of differentiated iSNs, with 1 μM concentrations resulting in 1% to 2% of cells responding,49 and 10 μM concentrations resulting in 2% to 10% of cells responding.41 Likewise, we found very few calcium responders to 0.1 μM capsaicin (Figure 2C; 0.67%-5.26%) and 1 μM capsaicin (0.90%-6.45%), and more responses to 10 μM capsaicin (12.99%-14.55%). We did note lower peaks in Fura-2 ratios above baseline when comparing 1 μM responses (Figure 2D; 64% increase above baseline) with responses to 10 μM (396% above baseline) and 50 μM capsaicin (522% above baseline).
Functional characterization of HC and SCD iSNs using traditional and high-throughput calcium assays. HC and SCD iSNs respond to 50 mM KCl, 100 μM glutamate, and 50 μM ab-meATP in traditional calcium imaging setup (panels A and B show representative traces). (C) Concentration curve shows that HC and SCD iSNs have few calcium responders to 0.1 and 1 μM capsaicin (subthreshold), identifying 10 μM capsaicin as appropriate to capture iSN response consistent with previous studies (panel D shows representative traces). (E) Schematic of Fluo4-NW calcium flux assay adapted to capture population responses of entire wells of iSNs to allow for high-throughput investigation of iSN sensitization. (F) Concentration curve using population-based Fluo-4NW assay allows for increased capture of subthreshold (0.1 μM, blue; 1 μM, pink) capsaicin although with low magnitude responses (F, right). Low-concentration (10 μM, teal) and high-concentration (50 μM, purple) capsaicin agonism resulted in consistent and high calcium response rates with increased magnitude (1-way ANOVA, ∗P < .05, ∗∗∗P < .0005). Consistent with calcium imaging data, Fluo-4NW assay shows no significant differences between HC and SCD iSN response to 50 mM KCl (G), 100 μM glutamate (H), and 50 μM ab-meATP (I), 10 μM capsaicin (J), or 50 μM capsaicin at baseline (K) (Student t tests; ns, not significant). Points in the violin plots represent individual wells of iSNs, and means are represented as a dashed line.
Functional characterization of HC and SCD iSNs using traditional and high-throughput calcium assays. HC and SCD iSNs respond to 50 mM KCl, 100 μM glutamate, and 50 μM ab-meATP in traditional calcium imaging setup (panels A and B show representative traces). (C) Concentration curve shows that HC and SCD iSNs have few calcium responders to 0.1 and 1 μM capsaicin (subthreshold), identifying 10 μM capsaicin as appropriate to capture iSN response consistent with previous studies (panel D shows representative traces). (E) Schematic of Fluo4-NW calcium flux assay adapted to capture population responses of entire wells of iSNs to allow for high-throughput investigation of iSN sensitization. (F) Concentration curve using population-based Fluo-4NW assay allows for increased capture of subthreshold (0.1 μM, blue; 1 μM, pink) capsaicin although with low magnitude responses (F, right). Low-concentration (10 μM, teal) and high-concentration (50 μM, purple) capsaicin agonism resulted in consistent and high calcium response rates with increased magnitude (1-way ANOVA, ∗P < .05, ∗∗∗P < .0005). Consistent with calcium imaging data, Fluo-4NW assay shows no significant differences between HC and SCD iSN response to 50 mM KCl (G), 100 μM glutamate (H), and 50 μM ab-meATP (I), 10 μM capsaicin (J), or 50 μM capsaicin at baseline (K) (Student t tests; ns, not significant). Points in the violin plots represent individual wells of iSNs, and means are represented as a dashed line.
To better capture capsaicin responders, we used a calcium flux assay (Fluo4-NW) to measure total intracellular calcium in response to agonist treatment (Figure 2E). By measuring changes in the entire population of cells in each well, we captured calcium flux increases in response to 0.1 and 1 μM capsaicin in 40% to 50% of stimulated wells (Figure 2F, left), and consistently high response rates to 10 μM (88.9%) and 50 μM (90%) capsaicin. The magnitude of these responses, calculated as the percent increase of Fluo-4 fluorescence after agonist stimulation compared with background fluorescence measured before agonist (Figure 2F, right; supplemental Figure 4), increased in 10 μM (8.87%) and 50 μM (16.25%) capsaicin conditions compared with 0.1 μM (3.35%) and 1 μM (2.41%) capsaicin conditions. Using these data, we labeled 1 μM capsaicin as subthreshold, 10 μM capsaicin as low-concentration, and 50 μM capsaicin as high-concentration, for future experiments.
Using the Fluo4-NW assay, we again found no differences in HC and SCD iSN population-based response to 50 mM KCl (Figure 2G; t test, P > .05), 100 μM glutamate (Figure 2H; P > .05), or 50μ M ab-meATP (Figure 2I; P > .05). When comparing HC and SCD iSNs responses to 10 and 50 μM capsaicin, we found no differences in response based on disease state (Figure 2J-K; P > .05). These data confirm that the iSNs respond to stimulation with an increase in intracellular calcium associated with neuronal firing.
SCD iSNs exhibit differential sensitization to electrical stimulation and exposure to human plasma
In SCD, a minimal blood nerve barrier at the level of DRGs becomes compromised and leaky,58 thereby allowing circulating ligands access to receptors and ion channels on peripheral neuronal processes. Therefore, we treated HC and SCD iSNs with age- and race-matched plasma taken from healthy individuals without SCD (HC plasma, n = 4), individuals with SCD during baseline health (SS BL plasma, n = 4), and those same individuals with SCD during hospitalization for acute pain (SS IP, n = 4). Initially, we found no differences between the plasma-treated HC and SCD iSNs in response to 50 mM KCl (Figure 3A; supplemental Figure 3), 100 μM glutamate (Figure 3B), and 50 μM ab-meATP (Figure 3C; 2-way ANOVA, P > .05). However, SCD iSNs did show a significant increase in response to 10 μM capsaicin after SS IP plasma treatment compared with HC iSNs (Figure 3D; P = .0240). This difference was not present in the 50 μM capsaicin condition (Figure 3E; P > .05), suggesting we had reached a maximal threshold response. We also found that SS IP plasma increased SCD iSN response to 100 μM glutamate (Figure 3F, left; 2-way ANOVA, P = .0435) and 10 μM capsaicin (Figure 3F, right; P = .0266) compared with untreated SCD iSNs. HC iSNs did not show these same effects but did have increased response to 50 μM ab-meATP after SS IP plasma treatment compared with untreated SCD iSNs (P = .0405) and HC plasma–treated conditions (P = .0089). These data reveal possible intrinsic differences within SCD iSNs that may contribute to acute pain associated with vaso-occlusive events.
HC and SCD iSNs are differentially sensitized to electrical and agonist stimulation. HC and SCD iSNs treated with plasma samples from healthy patients (HC), patients with SCD at baseline (SS BL), or patients with SCD experiencing an acute pain crisis (SS IP) show changes in functional response to agonists. No significant differences in response to KCl (A), glutamate (B), or ab-meATP (C) were found between HC and SCD iSNs after any plasma treatments (2-way ANOVA; ns, not significant). (D) Treatment of SCD iSNs with SS IP plasma did significantly increase response to 10 μM capsaicin compared with HC iSN response after SS IP plasma treatment (2-way ANOVA, ∗P < .05). (E) No differences found between HC and SCD iSN response to 50 μM capsaicin after plasma treatment (2-way ANOVA, ns). (F) Data from panels B through D represented to compare effects of plasma on iSN response. SS IP plasma significantly increased SCD iSN response to glutamate (F, left) and 10 μM capsaicin (F, right) compared with untreated (UTX) SCD iSNs but in HC iSNs significantly increased response to ab-meATP only (F, middle); 2-way ANOVA, ∗P < .05, ∗∗P < .005. (G) SCD iSNs show increased intrinsic membrane excitability. Relationship between action potential firing and injected current (2-way repeated-measures ANOVA, significant interaction between genotype and current injection F(19,341) = 1.892, ∗P = .0139; Bonferroni multiple comparison at 190 pA HC vs SCD, ∗P = .0427); n = 2 independent differentiations. (H) Representative waveforms show current-evoked action potential firing from each treatment group. Calibration: 10 mV, 100 ms.
HC and SCD iSNs are differentially sensitized to electrical and agonist stimulation. HC and SCD iSNs treated with plasma samples from healthy patients (HC), patients with SCD at baseline (SS BL), or patients with SCD experiencing an acute pain crisis (SS IP) show changes in functional response to agonists. No significant differences in response to KCl (A), glutamate (B), or ab-meATP (C) were found between HC and SCD iSNs after any plasma treatments (2-way ANOVA; ns, not significant). (D) Treatment of SCD iSNs with SS IP plasma did significantly increase response to 10 μM capsaicin compared with HC iSN response after SS IP plasma treatment (2-way ANOVA, ∗P < .05). (E) No differences found between HC and SCD iSN response to 50 μM capsaicin after plasma treatment (2-way ANOVA, ns). (F) Data from panels B through D represented to compare effects of plasma on iSN response. SS IP plasma significantly increased SCD iSN response to glutamate (F, left) and 10 μM capsaicin (F, right) compared with untreated (UTX) SCD iSNs but in HC iSNs significantly increased response to ab-meATP only (F, middle); 2-way ANOVA, ∗P < .05, ∗∗P < .005. (G) SCD iSNs show increased intrinsic membrane excitability. Relationship between action potential firing and injected current (2-way repeated-measures ANOVA, significant interaction between genotype and current injection F(19,341) = 1.892, ∗P = .0139; Bonferroni multiple comparison at 190 pA HC vs SCD, ∗P = .0427); n = 2 independent differentiations. (H) Representative waveforms show current-evoked action potential firing from each treatment group. Calibration: 10 mV, 100 ms.
To further define intrinsic differences between SCD iSNs and HC iSNs, we used patch clamp electrophysiology and found stimulus-induced sensitization in SCD iSNs compared with HC iSNs. Current-evoked intrinsic excitability was substantially increased at 190 pA current injection for SCD iSNs relative to HC iSNs (Figure 3G-H), but there was no significant change in resting membrane potential (average ± standard error of the mean, HC iSNs: −61.1 ± 2.0 mV; SCD iSNs: −57.5 ± 3.0 mV). Together, these data show that SCD iSNs have altered intrinsic excitability compared with HC iSNs that can be further affected by extrinsic exposure to plasma-derived factors and may contribute to neuronal sensitivity and pain in SCD.
Hb transcript expression is not associated with disease or oxidative stress in iSNs
Because SCD is characterized by a mutation in the Hb gene and recent studies have discovered unexpected expression of Hb in neuronal cultures,59-62 we measured transcript expression of the Hb-α (HBA) and Hb-β (HBB) chains. Both HBA and HBB transcripts were expressed at similar levels in HC and SCD iSNs (Figure 4A; ANOVA, P > .05). These transcript levels were also not differentially expressed after treatment with HC, SS BL, or SS IP plasma (Figure 4B; 2-way ANOVA, P > .05). Although there is evidence linking Hb expression in neurons to expression of genes associated with oxidative phosphorylation and mitochondrial function,59 targets involved in protection against oxidative stress63 were not significantly different between HC and SCD iSNs with or without plasma treatments (Figure 4C; 2-way ANOVA, P > .05).
Investigating the roles of Hb, ETRa, and CCL2 in SCD iSN response to painful stimuli. (A) Unexpected transcript production for both Hbα (HBA) and Hbβ (HBB) subunits in iSNs show no significant differences in expression between HC and SCD iSNs. (B) Hb transcript levels do not change when iSNs are treated with HC, SS BL, or SS IP plasma. (C) No significant differences in transcripts to indicate oxidative stress (catalase, HMOX1, and Nqo1) at baseline or with plasma treatments in HC and SCD iSNs. (D) Schematic of proposed roles of ETRa and CCL2 in SCD pathogenesis using a human iPSC model and connections to previously published mechanisms. Findings established using this iSN model are shown in red; purple arrows are suggested connections to previously published data (gray boxes). Treatment of iSNs with 100 ng/mL recombinant human CCL2 did not induce sensitivity of iSNs to agonists but did increase variability of SCD iSN responses compared with HC iSNs (t tests, ns) (E), whereas treatment of iSNs with 100 ng/mL recombinant human ET1 induced significantly higher responses to 50 mM KCl, 100 μM glutamate, and 10 μM capsaicin in SCD iSNs than in HC iSNs (t tests, ∗P < .05 [F]). Pretreatment with 100 μM BQ123 (ETRa inhibitor) and 100 μM BQ788 (ETRb inhibitor) reduced the effect of ET1 when paired with 10 μM capsaicin agonism in both HC (G) and SCD iSNs (ANOVA, ∗∗∗∗P < .0001) (H). Pretreatment with both ETRa and ETRb inhibitors significantly reduce responses to 10 μM capsaicin in HC (I) and SCD iSNs (J) even with the addition of HC, SS BL, and SS IP plasma treatment (ANOVA, ∗P < .05, ∗∗P < .005, and ∗∗∗∗P < .0001). Specifically, the increased sensitivity of SCD iSNs to 10 μM capsaicin after SS IP plasma was significantly reduced by ETRa and ETRb inhibition (ANOVA, ∗∗∗∗P < .0001). (K) Post-hoc analyses reveal high degree of variability and abnormal baseline intracellular calcium levels in SCD iSNs compared with HCs (t test, ∗∗P < .01).
Investigating the roles of Hb, ETRa, and CCL2 in SCD iSN response to painful stimuli. (A) Unexpected transcript production for both Hbα (HBA) and Hbβ (HBB) subunits in iSNs show no significant differences in expression between HC and SCD iSNs. (B) Hb transcript levels do not change when iSNs are treated with HC, SS BL, or SS IP plasma. (C) No significant differences in transcripts to indicate oxidative stress (catalase, HMOX1, and Nqo1) at baseline or with plasma treatments in HC and SCD iSNs. (D) Schematic of proposed roles of ETRa and CCL2 in SCD pathogenesis using a human iPSC model and connections to previously published mechanisms. Findings established using this iSN model are shown in red; purple arrows are suggested connections to previously published data (gray boxes). Treatment of iSNs with 100 ng/mL recombinant human CCL2 did not induce sensitivity of iSNs to agonists but did increase variability of SCD iSN responses compared with HC iSNs (t tests, ns) (E), whereas treatment of iSNs with 100 ng/mL recombinant human ET1 induced significantly higher responses to 50 mM KCl, 100 μM glutamate, and 10 μM capsaicin in SCD iSNs than in HC iSNs (t tests, ∗P < .05 [F]). Pretreatment with 100 μM BQ123 (ETRa inhibitor) and 100 μM BQ788 (ETRb inhibitor) reduced the effect of ET1 when paired with 10 μM capsaicin agonism in both HC (G) and SCD iSNs (ANOVA, ∗∗∗∗P < .0001) (H). Pretreatment with both ETRa and ETRb inhibitors significantly reduce responses to 10 μM capsaicin in HC (I) and SCD iSNs (J) even with the addition of HC, SS BL, and SS IP plasma treatment (ANOVA, ∗P < .05, ∗∗P < .005, and ∗∗∗∗P < .0001). Specifically, the increased sensitivity of SCD iSNs to 10 μM capsaicin after SS IP plasma was significantly reduced by ETRa and ETRb inhibition (ANOVA, ∗∗∗∗P < .0001). (K) Post-hoc analyses reveal high degree of variability and abnormal baseline intracellular calcium levels in SCD iSNs compared with HCs (t test, ∗∗P < .01).
ET1 signaling through ETRs may contribute to SCD iSN sensitization after plasma treatment
Next, we hypothesized that other intrinsic differences between HC and SCD iSNs may contribute to altered calcium responses in SCD iSNs (Figure 4D). Previous studies have shown that plasma from individuals with SCD contains increased levels ET1.64-69 Signaling of ET1 through increased ETRa receptor expression in SCD iSNs can contribute to decreased nitric oxide release70,71 contributing to vasoconstriction,72,73 increased intracellular calcium,73,74 and increased expression of ion channels.64,75-77 Moreover, increased CCL2 production by SCD iSNs can result in increased neuronal excitability,78,79 ion channel expression and function,80 release of glutamate,81 and recruitment of immune cells resulting in inflammation.54,82 Therefore, we added 100 ng/mL of recombinant human CCL2 (Figure 4E) and ET1 (Figure 4F) to HC and SCD iSN cultures. ET1 significantly increased calcium flux of the SCD iSNs in response to 50 mM KCl (P = .0333), 100 μM glutamate (P = .0186), and 10 μM capsaicin (P = .0333) compared with HC iSNs. CCL2 did not induce these direct increases in calcium flux (P > .05), although it did increase the range of calcium responses of SCD iSN to glutamate and 10 μM capsaicin (Figure 4E).
To determine whether the effect of SS IP plasma on SCD iSNs associated with signaling through ETRa, we first pretreated iSNs with 100 μM of ETRa or ETRb antagonist for 24 hours before addition of ET1. As expected, both ETRa and ETRb inhibition significantly reduced the effects of ET1 on HC (Figure 4G; 2-way ANOVA, P < .0001) and SCD iSNs (Figure 4H; 2-way ANOVA, P < .0001) when stimulated with 10 μM capsaicin. We then applied HC, SS BL, and SS IP plasma onto HC (Figure 4I) and SCD iSNs (Figure 4J) pretreated with ETRa or ETRb antagonists. Neither ETRa nor ETRb inhibition significantly altered baseline HC iSN response to 10 μM capsaicin (Figure 4I; 2-way ANOVA, P > .05, P > .05), although ETRb inhibition did decrease HC calcium response to both SS BL (P = .0264) and SS IP plasma (P = .0220) compared with untreated HC iSNs. ETRa inhibition also decreased HC iSN response to SS IP plasma compared with untreated HC iSNs (P = .0425) but, surprisingly, did not significantly decrease HC iSN response to SS BL plasma (P > .05). Similarly, both ETRa and ETRb inhibition significantly reduced SCD iSN response to 10 μM capsaicin when treated with SS IP plasma (Figure 4J; 2-way ANOVA, P < .0001, P < .0001) compared with untreated iSNs. Unlike HC iSNs, inhibition of ETRa (P = .0405) and ETRb (P = .0265) did significantly reduce the effect of SS BL plasma on SCD iSN response to 10 μM capsaicin, although the range of responses was again greater when inhibiting ETRa compared with ETRb. Furthermore, ETRa inhibition significantly reduced responses of SCD iSNs to 10 μM capsaicin at baseline (P = .0386) and when treated with HC plasma (P = .0026), suggesting a role for this receptor in baseline sensitization of SCD iSNs.
Post-hoc analyses revealed variable calcium response levels and a significant number of SCD iSNs with abnormally high baseline calcium levels compared with HC iSNs (Figure 4K; P = .0024) supporting the idea that some SCD iSNs are residing in an abnormal state, thereby potentially contributing to increased sensitivity to circulating plasma factors during acute SCD pain.
Polyamines found in SS patient plasma sensitize SCD iSNs to subthreshold capsaicin
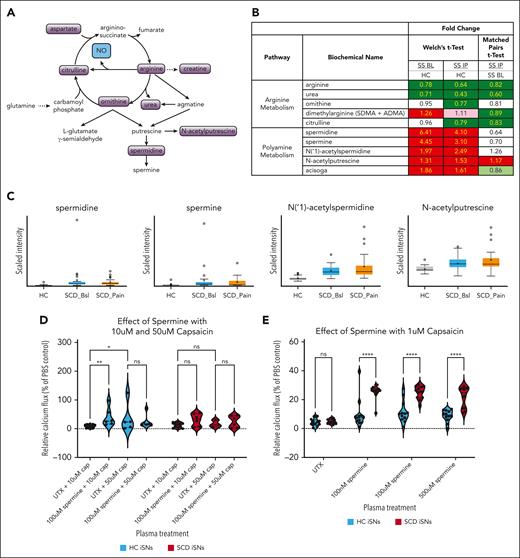
Although ETR inhibition did suppress the influence of ET1 on iSN calcium flux, the effect of ET1 treatment was neither capsaicin specific nor SCD iSN specific (Figure 4I-J) and do not explain why SCD iSNs are particularly sensitized to 10 μM capsaicin by SS IP plasma (Figure 3D). Therefore, we compared the metabolomic profiles of HC, SS BL, and SS IP plasma samples (n = 25 per group, supplemental Table 5). We chose to focus on arginine metabolism pathways, because arginine is known to be altered in SCD and decreased levels are associated with increased pain in patients with SCD.83-85 Spermidine is a polyamine produced as a metabolite of arginine metabolism through ornithine consumption86 (Figure 5A). Both ornithine and urea were decreased in SS IP plasma (Figure 5B), which may be explained by increased consumption of ornithine for polyamine synthesis. In support of this, the polyamines spermidine, spermine, N(‘1)-acetylspermidine, N-acetylputrescine, and acisoga had significantly higher expression in SS BL and SS IP plasma than in HC plasma (Figure 5B-C; Welch t test, P < .05).
SCD iSNs show increased sensitivity to capsaicin in the presence of spermine. (A) Schematic of arginine and polyamine metabolism relevant for specific biochemicals related to pain and inflammatory state in SCD. (B) Fold-change expression of biochemicals involved in these pathways indicate upregulation of polyamines, including spermine and its metabolites, in both SS BL and SS IP plasma compared with in HCs (Welch t test; dark red and dark green boxes, P < .05; light red and light green, P > .05 but P < .10). (C) Box plots of selected targets from panel B showing quartiles and medians of distribution for metabolite scaled intensity. (D) Significantly increased sensitivity of HC iSNs and trend for increased sensitivity of SCD iSNs (P = .0949) to 10 μM capsaicin after a 30-minute 100 μM-spermine treatment (2-way ANOVA, ∗ P < .05, ∗∗P < .005). (E) Interestingly, significant increase SCD iSN response to previous subthreshold (1 μM) capsaicin compared to HC iSNs was induced by treatment with 100 nM, 100 μM, and 500 μM spermine concentrations (2-way ANOVA, ∗∗∗∗P < .0001).
SCD iSNs show increased sensitivity to capsaicin in the presence of spermine. (A) Schematic of arginine and polyamine metabolism relevant for specific biochemicals related to pain and inflammatory state in SCD. (B) Fold-change expression of biochemicals involved in these pathways indicate upregulation of polyamines, including spermine and its metabolites, in both SS BL and SS IP plasma compared with in HCs (Welch t test; dark red and dark green boxes, P < .05; light red and light green, P > .05 but P < .10). (C) Box plots of selected targets from panel B showing quartiles and medians of distribution for metabolite scaled intensity. (D) Significantly increased sensitivity of HC iSNs and trend for increased sensitivity of SCD iSNs (P = .0949) to 10 μM capsaicin after a 30-minute 100 μM-spermine treatment (2-way ANOVA, ∗ P < .05, ∗∗P < .005). (E) Interestingly, significant increase SCD iSN response to previous subthreshold (1 μM) capsaicin compared to HC iSNs was induced by treatment with 100 nM, 100 μM, and 500 μM spermine concentrations (2-way ANOVA, ∗∗∗∗P < .0001).
Spermine has been shown to directly activate TRPV1 and potently sensitize TRPV1 to capsaicin activation.87 Therefore, we examined whether spermine pretreatment would sensitize iSNs to 10 μM or 50 μM capsaicin (Figure 5D). Treatment with 100 μM spermine significantly increased the HC iSN calcium response (2-way ANOVA, P = .0038) and induced a trending increase in the SCD iSNs (P = .0949) to 10 μM capsaicin. No increase occurred in the 50 μM capsaicin condition in HC (P > .05) or SCD iSNs (P > .05), suggesting a possible ceiling effect at this high capsaicin concentration. Strikingly, we found that, compared with HC iSNs, spermine treatment significantly increased the calcium response of SCD iSNs to the subthreshold concentration of 1 μM capsaicin (Figure 5E, 2-way ANOVA, P < .0001). Together, these data show that plasma-derived factors can sensitize SCD iSNs to capsaicin and may contribute to the neuronal sensitivity and pain in SCD.
Discussion
SCD iPSCs have been used to study erythrocyte biology,88-90 but here we used SCD iPSCs to study intrinsic and dynamic mechanisms of nociception in SCD. Our data confirm that iSNs are morphologically and functionally consistent with in vivo sensory neurons regarding channel and receptor expression91 and electrophysiological function.37,39 Although we found no differences in marker expression or select transcripts between HC and SCD iSNs, a more unbiased RNA sequencing approach would be needed to fully assess potential differences between SCD and HC iSNs. Nevertheless, we did find that SCD iSNs had altered baseline calcium levels, increased excitability compared with HC iSNs, and were sensitized to nociceptive stimuli by patient-derived SCD plasma suggesting a novel finding that SCD iSNs have intrinsic alterations in excitability that may contribute to pain phenotypes.
Although ET-1 induces oxidative stress through both ETRa and ETRb signaling in other cell types,92-94 we did not identify any changes in oxidative stress markers after exposure of HC and SCD iSNs to patient-derived SCD plasma. Additionally, although increased CCL2 is associated with glutamate signaling in sensory neurons,81 application of CCL2 increased response variability but did not lead to significant sensitization of SCD iSNs response compared with HC iSN responses. These pathways may be more driven by extrinsic factors in vivo or require interactions of sensory neurons with other cell types.
Beyond ET1 and CCL2, we hypothesized other factors in patient-derived SCD plasma sensitize iSNs. Our metabolomics data revealed several dysregulated factors involved in arginine and polyamine metabolism in patient-derived SCD plasma compared to plasma from HCs but also identified differences between baseline and acute pain state of patients with SCD. Further investigation into these metabolites could inform ongoing clinical trials seeking to reduce SCD pain through arginine supplementation.85 We found increased SCD iSN response after spermine treatment to 1 μM capsaicin, a concentration that in untreated iSNs does not elicit increased intracellular calcium. This finding indicates an intrinsic mechanism by which SCD iSNs exhibit increased sensitivity to TRPV1 activation and support our previous findings that nociceptor TRPV1 is sensitized in mice with SCD.24 Our metabolomic analysis focused on polyamines, but future experiments could assess both the plasma composition and transcriptional profiles of iSNs to determine additional mechanisms contributing to iSN sensitization.
The iSN platform has limitations, and in vivo studies may still be more appropriate to examine sensory neuron phenotypes or disease development across the lifespan. Although iSNs recapitulate phenotypes identified in mouse and primary human cultures, the concentration of capsaicin required to induce a robust calcium response in iSNs is higher than that required for primary DRG cultures in either mouse or human.38 The purified nature of iSN cultures is an advantage to studying intrinsic cell-specific mechanisms of sensitization, but interactions with other cell types likely contribute to maturation and sensitization for native sensory neurons in vivo. Our iSN cultures do not appear to contain peripheral glia, but other non-capsaicin responsive neurons in the culture may contribute to the SCD iSN sensitivity. Future experiments could better investigate individual neuronal cell types or use established coculture techniques to grow iSNs with other relevant interacting cell types within the in vivo milieu. Because a limited number of SCD iPSC lines are available, as well as age- and race-matched controls for these lines, we cannot fully address patient heterogeneity or differences based on sex nor can we fully generalize these findings to all individuals with SCD. Nevertheless, these data are a foundation to assess molecular mechanisms in iSNs that may be relevant to pain neurobiology. Although SCD affects males and females equally, recent analyses show that females have significantly worse pain frequency and severity,95 indicating that future in-depth analyses are necessary to provide additional mechanistic insight.
In summary, our data indicate that SCD iSNs exhibit intrinsic excitability and sensitization to external blood-derived stimuli compared with HC iSNs, which suggests a combination of central and peripheral mechanisms are involved in SCD pain.
Acknowledgments
The authors thank and acknowledge individuals with and without SCD who contributed to this study, and also thank Bhavya Dharanikota and Benjamin O’Brien for technical assistance with calcium imaging. Figures 2E and 4D, supplemental Figure 1, and the visual abstract were created with BioRender.com.
This work was supported by funding from the National Institutes of Health (NIH), National Institute of Neurological Disorders and Stroke (R01NS070711 [C.S.], R37NS108278 [C.S.]), NIH, National Heart, Lung, and Blood Institute (1K23HL114636-01A1 [A.M.B.], 1R01HL142657-01 [A.M.B.]), and Advancing a Healthier Wisconsin Endowment (A.D.E., C.S., and A.M.B.).
Authorship
Contribution: R.L.A., E.W., V.E., A.B., and O.I. performed and analyzed experiments; J.H. coordinated plasma sample preparation and delivery; all authors designed experiments and interpreted data; A.M.B., C.S., and A.D.E. supervised the study and provided funding; R.L.A. wrote the manuscript; R.L.A., E.W., V.E., O.I., A.B., and D.N.T. created figures; and all authors edited and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Allison D. Ebert, Department of Cell Biology, Neurobiology and Anatomy, Medical College of Wisconsin, 8701 Watertown Plank Rd, Milwaukee, WI 53226; email: aebert@mcw.edu.
References
Author notes
Original data are available on request from the corresponding author, Allison D. Ebert (aebert@mcw.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.




![Investigating the roles of Hb, ETRa, and CCL2 in SCD iSN response to painful stimuli. (A) Unexpected transcript production for both Hbα (HBA) and Hbβ (HBB) subunits in iSNs show no significant differences in expression between HC and SCD iSNs. (B) Hb transcript levels do not change when iSNs are treated with HC, SS BL, or SS IP plasma. (C) No significant differences in transcripts to indicate oxidative stress (catalase, HMOX1, and Nqo1) at baseline or with plasma treatments in HC and SCD iSNs. (D) Schematic of proposed roles of ETRa and CCL2 in SCD pathogenesis using a human iPSC model and connections to previously published mechanisms. Findings established using this iSN model are shown in red; purple arrows are suggested connections to previously published data (gray boxes). Treatment of iSNs with 100 ng/mL recombinant human CCL2 did not induce sensitivity of iSNs to agonists but did increase variability of SCD iSN responses compared with HC iSNs (t tests, ns) (E), whereas treatment of iSNs with 100 ng/mL recombinant human ET1 induced significantly higher responses to 50 mM KCl, 100 μM glutamate, and 10 μM capsaicin in SCD iSNs than in HC iSNs (t tests, ∗P < .05 [F]). Pretreatment with 100 μM BQ123 (ETRa inhibitor) and 100 μM BQ788 (ETRb inhibitor) reduced the effect of ET1 when paired with 10 μM capsaicin agonism in both HC (G) and SCD iSNs (ANOVA, ∗∗∗∗P < .0001) (H). Pretreatment with both ETRa and ETRb inhibitors significantly reduce responses to 10 μM capsaicin in HC (I) and SCD iSNs (J) even with the addition of HC, SS BL, and SS IP plasma treatment (ANOVA, ∗P < .05, ∗∗P < .005, and ∗∗∗∗P < .0001). Specifically, the increased sensitivity of SCD iSNs to 10 μM capsaicin after SS IP plasma was significantly reduced by ETRa and ETRb inhibition (ANOVA, ∗∗∗∗P < .0001). (K) Post-hoc analyses reveal high degree of variability and abnormal baseline intracellular calcium levels in SCD iSNs compared with HCs (t test, ∗∗P < .01).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/143/20/10.1182_blood.2023022591/2/m_blood_bld-2023-022591-gr4.jpeg?Expires=1765981867&Signature=N7fvKkKzNaEo-cPJKSQajBhQtLiFWj7DCLAXRUDVU2SumkwlbGqAthdZatX~Bo1aScSLIi9rsVhEBCiozHY~cv7Xqp-RbteuhOf1q6FoTycWwI~-m09HYMeQWUqzOsiCCNZYhypGtNrLJKFINP46iIe~WrwQ0WyqXRYugV3DumMwn9hq5yDSzaz1Aek7NKILrrvEZPyeagaGlpIWEKQb3wg5bFBYzPhlNf2DicHwh2zq-kjXXo5W9brTS1okNnloqRmwyaCOzm5R9fXcSDyk2~W8EEE8eGDk9yQ3jWoS65rJQ-Hk8tBq1Uvq7daPGX3jrMgiVyAEN0g5C0d80M4Xow__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal