In this issue of Blood, Touzeau et al report the results of the phase 2 IFM 2018-04 trial of quadruplet induction and double autologous stem cell transplantation (ASCT) in patients with high-risk (HR) multiple myeloma (MM).1 Induction therapy included 6 cycles of quadruplet therapy with daratumumab, carfilzomib, lenalidomide, and dexamethasone (D-KRd), followed by melphalan ASCT. Thereafter, patients received 4 additional D-KRd cycles and a second melphalan ASCT, followed by 2 years of planned daratumumab-lenalidomide (dara-len) maintenance. Of 50 enrolled patients, 36 received both ASCTs and (as of publication) 5 patients are now on observation after having completed dara-len maintenance; with this approach, 2-year progression-free survival (PFS) ranged from 80% to 85%. Although infections such as COVID-19 were common and were responsible for both treatment-related deaths, only a single patient developed reversible cardiac dysfunction with carfilzomib.
The IFM 2018-04 trial adds another paradigm to the field’s growing list of approaches for treating HR MM in ASCT-eligible patients. The MASTER trial, which used up to 12 cycles of D-KRd with a single ASCT, allowed for treatment discontinuation after achievement of sustained measurable residual disease (MRD) negativity; in this trial, 2-year PFS was 84% pooled across patients with 1 and ≥2 HR features.2 The GMMG-CONCEPT trial, which employed ≈3 years’ worth of isatuximab (Isa) plus KRd (carfilzomib, lenalidomide, dexamethasone), demonstrated a similar 2-year PFS of 78.3% among ASCT-eligible patients.3 The phase 2 OPTIMUM MUKnine trial, which only enrolled patients with ultra-HR MM (≥2 HR features), employed a phased deescalation of daratumumab, cyclophosphamide, bortezomib, lenalidomide, and dexamethasone (D-CVRd) and single ASCT over several years; here, 2-year PFS was 77.9%.4 Many other approaches to treating HR MM have been studied as well, ranging from changes in induction to changes in peri-ASCT conditioning or maintenance.5
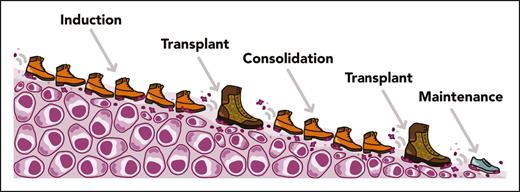
Regardless of cytogenetic risk, quadruplet induction has now become the standard of care for ASCT-eligible patients in many countries based on a growing number of studies, including the recently published phase 3 PERSEUS trial.6 For patients with HR cytogenetics, the above-mentioned trials suggest that giving more treatment for longer is a key ingredient for response durability. But which metaphorical sequencing of footwear (see figure) carries the most force to stamp out HR MM over time? The principle that serial ASCTs may offer a benefit in HR MM is not new. In the BMT-CTN 0702 STAMINA trial, a post hoc analysis of patients with HR MM who successfully received tandem ASCT showed improved 6-year PFS (44% vs 26%) vs those who received a single ASCT.7 However, in part because over a third of patients in the trial’s tandem ASCT arm did not receive a second ASCT as planned, the primary intent-to-treat analysis was negative. The IFM 2018-04 study instead separated the second ASCT by 4 D-KRd cycles from the first one, and only 1 of 42 patients who underwent transplant withdrew study consent before their second ASCT.1 Perhaps a delay between serial ASCTs to allow for functional recovery, rather than requiring a strict tandem approach, is a simple tactic to make this approach more practical.
The IFM 2018-04 approach to high-risk multiple myeloma. Visual illustration of the IFM 2018-04 trial, including induction with D-KRd and 2 ASCTs. Professional illustration by Somersault18:24.
The IFM 2018-04 approach to high-risk multiple myeloma. Visual illustration of the IFM 2018-04 trial, including induction with D-KRd and 2 ASCTs. Professional illustration by Somersault18:24.
One important caveat about second transplantation in this study is that 16% of participants (8 patients altogether) were not able to receive one or both planned ASCTs because of inadequate stem cell collection with cyclophosphamide and filgrastim. Although this was a phase 2 trial, it was powered for feasibility, as defined by a 70% success rate with regard to patients receiving both transplantations. The study met its primary end point by only a single-patient margin, highlighting the importance of strategies to maximize stem cell yields during treatment with quadruplet induction. For patients with high disease burden or HR cytogenetics, stem cell collection after 3 to 4 cycles should be planned. Alternatively, plerixafor should be considered early as a rescue strategy if needed.8
So, should all patients with HR MM be receiving D-KRd, 2 transplants, and 2 years of dara-len maintenance? Not necessarily. D-KRd or Isa-KRd are attractive induction options based on the IFM 2018-04, MASTER, and GMMG-CONCEPT studies.1-3 However, whether carfilzomib truly outperforms bortezomib in the newly diagnosed HR setting is unknown. For ultra-HR patients in the MUKnine trial, D-CVRd with liberal dose reductions to maintain patients on therapy yielded impressive results.4 To better tailor the toxicities inherent to double transplantation, a previous IFM study published >20 years ago concluded that a second transplantation offers the most benefit only for patients who did not achieve a satisfactory response following the first ASCT.9 The ideal maintenance strategy in HR MM is also unclear. Doublets are almost certainly preferable over lenalidomide monotherapy; however, both proteasome inhibitors and monoclonal antibodies have demonstrated efficacy as partner agents. Finally, optimal maintenance duration is unknown: should we continue maintenance indefinitely as done in MUKnine,4 deescalate with a time-based threshold as done in IFM 2018-04 and GMMG-CONCEPT,1,3 deescalate based on sustained MRD negativity as done in MASTER,2 or deescalate based on a combination of these factors as done in the dara-len arm of PERSEUS?6 Perhaps, as shown in MASTER and tested in MUKnine, different pathways should be applied for patients with ultra-HR MM as opposed to only 1 HR feature.2,4
Further follow-up from these and future trials will be essential to answer these questions. At a broader level, further studies are sorely needed to better identify risk factors for “functional HR” MM. Preventing early relapse is the raison d’être of these intensified regimens, but over a third of patients with MM with early relapse do not have any HR cytogenetic abnormalities and thus would not ordinarily receive these types of approaches.10 Regardless, the excellent study by Touzeau et al demonstrates the feasibility of quadruplet therapy in HR MM and the staying power of double ASCT as an option in these scenarios. We are fortunate as a field to have a growing collection of footwear with which to stamp out HR MM. Even if prolonged induction and consolidation with ASCT cannot eradicate myeloma entirely, perhaps the incorporation of chimeric antigen receptor T-cell therapies and bispecific antibodies in coming years will help us discover the best way to finally give MM the boot.
Conflict-of-interest disclosure: R.B. has performed consulting for Bristol Myers Squibb, Caribou Biosciences, Genentech, Janssen, Legend, Pfizer, Sanofi, and SparkCures; and has received research support from Novartis and Pack Health. J.R.M. has performed consulting for Amgen, Bristol Myers Squibb, Janssen, Karyopharm, Pfizer, Sanofi, and Takeda.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal