In this issue of Blood, Othman et al have identified the level of molecular measurable residual disease (MRD) in peripheral blood after 2 cycles of intensive chemotherapy that could guide the indication for transplantation in patients with acute myeloid leukemia (AML) with NPM1 mutation.1
Historically, complete remission (CR) following intensive chemotherapy has been morphologically defined in patients with AML. However, more than 50% of patients in CR ultimately relapse because of a high burden of morphologically undetectable residual disease. This residual disease is now routinely detected by multiparameter flow cytometry (MFC), real-time quantitative polymerase chain reaction (qPCR), and more recently by next-generation sequencing.2 Many studies have shown that the greater the MRD, the higher the risk of relapse and death, and recent guidelines have included MRD in the response criteria.3 Achieving CR with negative MRD is therefore a major goal after first-line chemotherapy, and MRD status is used to guide subsequent treatments during consolidation, including allogeneic hematopoietic stem-cell transplantation (allo-HSCT). However, the prognostic weight of initial disease criteria vs MRD in defining whether a patient would benefit from transplantation has not yet been firmly established, especially in patients with intermediate-risk AML.
In this study, the investigators used data from 2 prospective, randomized, multicenter clinical trials to determine whether MRD could help better select patients who would benefit from allo-HSCT. They focused obviously on patients with NPM1 mutations because of its relatively high frequency and the possibility of tracking the mutant by qPCR. In the 2022 European LeukemiaNet (ELN) risk classification, patients with NPM1 mutations are mainly classified as favorable or intermediate depending on the presence of FLT3-internal tandem duplication (ITD) or exceptionally as adverse risk if high-risk cytogenetic abnormalities are present regardless of FLT3-ITD status.4 They show that, in terms of overall survival, patients with positive MRD in peripheral blood after 2 cycles of intensive chemotherapy did benefit from allo-HSCT, whereas those with undetectable MRD did not (although the relapse rate was greater if not transplanted in CR1). Importantly, multivariate analysis showed that this finding holds true in specific subgroups with a higher risk of relapse including patients with NPM1/DNMT3A/FLT3-ITD triple mutation or patients with a high FLT3-ITD allelic ratio. This result is important because it addresses the main issue raised several years ago by a landmark study from the same group, which had established the independent prognostic role of molecular MRD in NPM1-mutated AML.5 It will also likely improve the current 2022 ELN risk classification to guide allo-HSCT indications in intermediate-risk patients with NPM1 and FLT3-ITD comutations and, potentially, change practice in centers that traditionally transplant all patients with FLT3-ITD in CR1 regardless of NPM1 comutation or MRD status.
There are other interesting points in this study worth discussing. First, the number of patients aged over 60 years was too limited to generalize the main conclusion of the study to older patients. Second, patients who had positive MRD after 2 cycles of induction but became negative before allo-HSCT had a better outcome than patients who were positive at both time points, suggesting that consolidation treatments that target MRD more effectively could contribute to reducing the risk of posttransplant relapse. Third, it is noteworthy that these studies were carried out before the approval of midostaurin and quizartinib in AML with FLT3 mutations. These FLT3 inhibitors can induce a deeper molecular response in combination with chemotherapy, thereby potentially reducing the proportion of patients with a transplantation indication. They also alter the molecular composition of AML clones at relapse, with loss of the FLT3 mutation in 50% of cases, which must be taken into account when defining second-line therapy.6 Lastly, around 40% of patients with NPM1 mutations with negative MRD who were not transplanted in CR1 still relapsed, requiring salvage treatment including allo-HSCT in 60% of cases. Thus, although the overall survival of MRD-negative patients seems satisfactory, this comes at the price of a second sequence of treatment including allo-HSCT in a substantial number of patients. This also underlines that MRD-negative patients need to be carefully monitored by qPCR in blood, ideally every 4 to 6 weeks for 2 years according to current recommendations, and raises the issue of early therapeutic intervention in patients with confirmed molecular relapse, a new major therapeutic challenge for AML therapy.3 Indeed, a recent study showed better outcomes when patients with NPM1 mutation or core binding factor (CBF) AML are treated at the time of molecular relapse rather than morphological relapse.7 Currently, there is no consensus treatment for patients with molecular relapse before overt clinical relapse. Frontline allo-HSCT, salvage intensive chemotherapy, waiting for morphological relapse to screen molecular characteristics of the relapsing disease, or proposing a clinical trial may all be discussed with patients. Preliminary data also suggest that venetoclax and azacitidine induce a high rate of second molecular response with minimal toxicity, which is desirable before allo-HSCT.8,9 It is also conceivable that menin inhibitors may also play a role in this situation.
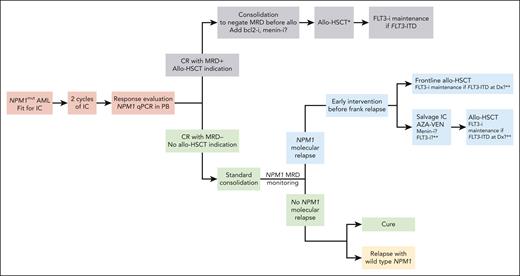
To conclude, this study shows that molecular MRD is probably the only criterion to use as an indication for allo-HSCT in patients with NPM1 mutations who are fit for intensive chemotherapy and provides clues for redesigning the global management of these patients (see figure). Whereas a similar approach may be advocated in CBF AML, it remains to be prospectively determined whether MRD monitoring by MFC can also be a reliable marker to drive allo-HSCT indications in other subtypes of AML.10
Management algorithm for AML with NPM1 mutations according to MRD. ∗In this study, no difference in overall survival was observed between myeloablative and reduced-intensity conditioning. ∗∗With current induction and consolidation therapy combining IC and FLT3-i, around 50% of relapses are FLT3-wild type, and therefore, using a FLT3-i in molecular relapse or posttransplant is still uncertain clinically. AZA-VEN, azacitidine-venetoclax; Dx, diagnosis; -i, inhibitors; IC, intensive chemotherapy; PB, peripheral blood.
Management algorithm for AML with NPM1 mutations according to MRD. ∗In this study, no difference in overall survival was observed between myeloablative and reduced-intensity conditioning. ∗∗With current induction and consolidation therapy combining IC and FLT3-i, around 50% of relapses are FLT3-wild type, and therefore, using a FLT3-i in molecular relapse or posttransplant is still uncertain clinically. AZA-VEN, azacitidine-venetoclax; Dx, diagnosis; -i, inhibitors; IC, intensive chemotherapy; PB, peripheral blood.
Conflict-of-interest disclosure: C.R. declares a consulting or advisory role with AbbVie, Amgen, Astellas, BMS, Boehringer, Jazz Pharmaceuticals, and Servier; received research funding from AbbVie, Amgen, Astellas, BMS, Iqvia, and Jazz Pharmaceuticals; and received support for attending meetings and/or travel from AbbVie, Novartis, and Servier.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal