T cell therapies, including chimeric antigen receptor (CAR) T cells, have revolutionized cancer therapy. However, impressive responses are limited to a subset of patients with hematological cancers and have not been unlocked in patients with solid tumors, which represent 90% of adult cancers. Adoptive T cell therapy in both treatment-resistant hematological malignancies and solid cancers is limited by a complex combination of factors, including poor in vivo persistence, immunosuppressive environmental factors, and T cell exhaustion. New solutions to overcome these limitations are needed to address the significant unmet need for effective cell therapies.
To overcome this engineering problem, we turned to spontaneously occurring T cell cancers. Over time, T cells, like other cells in the adult, acquire somatic mutations. When these mutations increase the fitness of the T cell, T cell clones emerge via positive selection. These T cell cancers develop genetic means to overcome similar cell-intrinsic and cell-extrinsic immunosuppressive factors facing anti-tumor T cells. Additionally, mutations from T cell cancers may unlock modifications not available by current screening approaches, because evolution employs any mutation available in nature. Like existing approaches, these mutations can modify gene expression by gene locus duplication or deletion. However, unlike current approaches, gain-of-function point mutations or translocations can be introduced that have unique, outsized effects unachievable by modulation of wild-type gene expression.
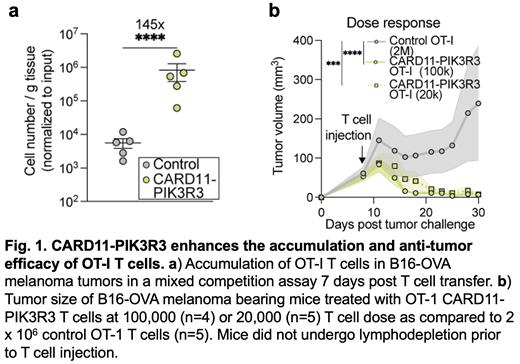
Here, we have generated a first-of-its-kind library of 71 mutations and 45 wild-type over-expression controls. We performed in vitro and in vivo screening to identify the effects of these mutations on CAR signaling, cytokine production, and accumulation in vivo in tumors. These screens converged on a gene fusion, CARD11-PIK3R3. Mechanistically, CARD11-PIK3R3 enhances CAR-dependent CARD11-BCL10-MALT1 complex signaling including NF-κB/AP-1 transcriptional activity and MALT1 substrate cleavage, in a BCL10 dependent manner. In vivo, CARD11-PIK3R3 transduced T cells exhibit enhanced intratumoral accumulation, effector functions, and therapeutic efficacy. Importantly, CARD11-PIK3R3 expression was sufficient to reduce T cell dose requirements and protect against tumor re-challenge in an immunotherapy refractory syngeneic model in the absence of lymphodepletion.
Our results indicate that exploiting naturally occurring mutations represents a promising approach to explore the extremes of T cell biology and discover how solutions derived from evolution of malignant T cells can improve a broad range of T cell therapies. Notably, the gene fusion we identified would be inaccessible through previous T cell screening efforts such as loss of function, CRISPR activation, or wild-type gene over-expression screens. Finally, our results implicate the CARD11-BCL10-MALT1 signalosome as a key regulator of therapeutic T cell function. Altogether, investigation of T cell lymphoma mutations in the context of adoptively transferred T cells has the promise to both improve cellular therapies and to elucidate new T cell biology.
Disclosures
Garcia:Moonlight Bio: Current Employment, Current equity holder in private company, Patents & Royalties. Daniels:Moonlight Bio: Current Employment, Current equity holder in private company, Patents & Royalties. Zhu:Moonlight Bio: Patents & Royalties. Roybal:Moonlight Bio: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees, Other: Co-founder, Patents & Royalties; Arsenal Biosciences: Consultancy, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees, Other: Co-founder; Gilead: Current equity holder in private company; Alaunos Oncology: Membership on an entity's Board of Directors or advisory committees. Choi:Moonlight Bio: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees, Other: Co-founder, Patents & Royalties.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal