Introduction and background
Key factors that relate to worse quality of life (QoL) in acute leukemia patients were identified in a global survey conducted by the Acute Leukemia Advocates Network (ALAN) such as age, gender, socioeconomic state, social functioning (e.g., worries about family and friends, feeling isolated, finances, etc.[ Pemberton-Whiteley et al, 2023]). These factors should be taken into consideration by healthcare professionals caring for acute leukemia patients to enable a robust support system.
The role of patient organizations (PO) in the care of the patient and family is becoming increasingly important, however, patients are often not aware of the existence of POs and physicians and providers are often unsure of the role and benefits of PO, which then leads to a delay in patients reaching out to POs (for information, education resources, support resources [support groups, online chat, peer-to-peer support, financial support, psychological support, carer support], etc.).
To date, there is limited evidence showing the importance of support offered to patients and their families. The ALAN, the Mayo Clinic and a national PO, are conducting a study that aims to assess both retrospectively and prospectively the benefits of active referrals to PO for patients with acute leukemia.
Hypothesis
We hypothesize that active referrals of adult patients with acute leukemia to PO at diagnosis has a positive impact on patient-reported outcomes and patient experience.
Methods
Here we present the study methodology that uses a quantitative method via administration of surveys at different timepoints.
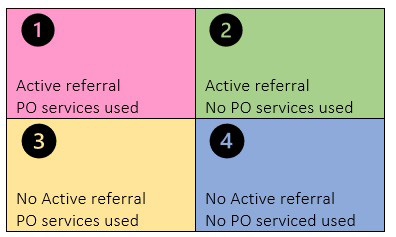
We plan to do both a retrospective and prospective evaluation of patients (target N=200) previously referred and new patients with a diagnosis of acute leukemia. There are 4 scenarios which are presented in Figure 1.
For patients already diagnosed and evaluated at Mayo Clinic (scenarios 3 and 4), we will contact them either via email or portal message to see if they are interested in the study. A study consent form will be sent to them. If they indicate interest and consent, they will be contacted to understand whether they were referred to or sought help from PO around the time of their diagnosis or subsequently, and a questionnaire will be sent to them to assess if PO services helped them in their disease or treatment course.
For newly diagnosed patients (scenarios 1 and 2), active referral to PO using PO referral online form will be provided and they will receive study consent form during their first visit at Mayo Clinic. A questionnaire will be sent to them to understand if they used the services from PO after the referral and if yes, to assess if PO services helped them in their disease or treatment course.
In addition, every month from diagnosis up to 6 months, all patients who consented to take part in the study will be asked to complete the validated HM-PRO QoL assessment tool (an instrument designed to measure patient-reported outcomes in those with hematological malignancies), to assess QoL and symptoms and change over time.
Conclusion
This is a pilot study aiming at building evidence on the importance of support offered to patients and their families and demonstrating the impact of an active referral at diagnosis.
1 Pemberton-Whiteley Z, Nier S, Geissler J, Wintrich S, Verhoeven B, Christensen RO, Salek S, Oliva EN, Ionova T, Bradley J. Understanding quality of life in patients with acute leukemia, a global survey. J Patient Cent Res Rev. 2023;10:21-30
Disclosures
Nier:Astra Zenaca: Other: Grant funding to the organization; Autolus: Other: Grant funding to the organization; BMS: Other: Grant funding to the organization; Cancell Therapeutics: Consultancy, Other: Grant funding to the organization; Daiichi Sankyo: Other: Grant funding to the organization; Incyte: Other: Grant funding to the organization; Jazz Pharmaceuticals: Other: Grant funding to the organization; Janssen: Consultancy, Other: Grant funding to the organization; Kite/Gilead: Other: Grant funding to the organization; Kura Oncology: Other: Grant funding to the organization; Novartis: Consultancy, Other: Grant funding to the organization; Otsuka: Consultancy, Other: Grant funding to the organization; Pleco Therapeutics: Other: Grant funding to the organization; Pfizer: Other: Grant funding to the organization; Roche: Other: Grant funding to the organization; Servier: Consultancy, Other: Grant funding to the organization; Abbvie: Consultancy, Other: Grant funding to the organization; Amgen: Other: Grant funding to the organization; Astellas: Consultancy, Other: Grant funding to the organization; Takeda: Other: Grant funding to the organization; Kyowa Kirin: Consultancy. Pemberton-Whiteley:Glycostem: Other: Grant funding to the organization; INO Therapeutics: Other: Grant funding to the organization; Zambon: Consultancy; Takeda: Other: Grant funding to the organization; Servier: Consultancy, Other: Grant funding to the organization; Seagan: Consultancy; Pfizer: Other: Grant funding to the organization; Otsuka: Consultancy, Other: Grant funding to the organization; Novartis: Consultancy, Other: Grant funding to the organization; Kyowa Kirin: Other: Grant funding to the organization; Pleco Therapeutics: Other: Grant funding to the organization; Kura Oncolovy: Other: Grant funding to the organization; Kite/Gilead: Other: Grant funding to the organization; Janssen: Consultancy, Other: Grant funding to the organization; Jazz: Other: Grant funding to the organization; Incyte: Other: Grant funding to the organization; Daiichi Sankyo: Other: Grant funding to the organization; Cancell Therapeutics: Consultancy, Other: Grant funding to the organization; BMS: Consultancy, Other: Grant funding to the organization; Autolus: Other: Grant funding to the organization; AstraZeneca: Consultancy, Other: Grant funding to the organization; Astellas: Consultancy, Other: Grant funding to the organization; Amgen: Other: Grant funding to the organization; Leukaemia Care: Current Employment; AbbVie: Consultancy, Other: Grant funding to the organization; Adaptive: Other: Grant funding to the organization.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal