Background: As the number of elderly patients referred to and undergoing allogeneic hematopoietic cell transplantation (alloHCT) continues to rise, there is a growing need for a comprehensive risk assessment model to accurately predict transplantation outcomes in this specific patient population. The widely used Hematopoietic Cell Transplantation-specific Comorbidity Index (HCT-CI) does not fully capture factors influencing transplant outcomes in elderly patients. This study aims to enhance risk stratification for elderly patients undergoing alloHCT by integrating additional functional, nutritional, and clinical indicators.
Methods: This is a retrospective study utilizing the data registry of HCT patients who received their treatment at UF Health Cancer Center. The inclusion criteria included all patients age > 65 years, who underwent their first alloHSCT between January 2012 and December 2022, regardless of graft type, conditioning regimen, donor type, or graft source. Patient data were extracted through chart review. The primary endpoint was 1-year Overall Survival (OS). Prognostic significance of each variable was assessed using Cox proportional hazard models. We subsequently used all variables to build a comprehensive prediction model to assess 1-year survival outcomes in elderly patients undergoing alloHCT. The predictive model's performance was measured by the Concordance-statistic (c-statistic).
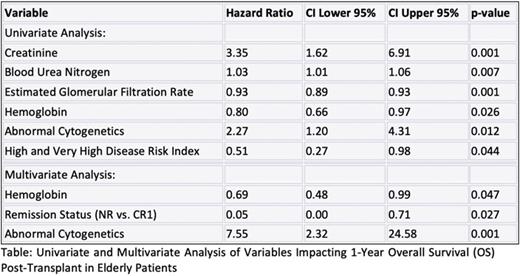
Results: A total of 69 patients satisfied the inclusion criteria and were included in the analysis. Median age was 69 years (range 66-81) and 65.2% were male. Unrelated donors were used in 66.6% of patients, while the remainder received grafts from related donors. Most patients received peripheral blood stem cell grafts (91%). Myeloid malignancies comprised the most common indication for alloHCT in this group of patients (89.8%). 59.4% had abnormal cytogenetics. 35 variables were considered in the analysis. In the univariate analysis, variables with impact on 1-year OS post-transplant included creatinine (HR 3.35, CI 1.62-6.91, p=0.001), blood urea nitrogen (HR 1.03, CI 1.01-1.06, p=0.007), estimated glomerular filtration rate (HR 0.93, CI 0.89-0.93, p=0.001), hemoglobin (HR 0.8, CI 0.66-0.97, p=0.02), abnormal cytogenetics (HR 2.27, CI 1.20-4.31, p=0.012) and high and very high disease risk index (HR 0.51, CI 0.27-0.98, p=0.04). Notably, both hemoglobin and cytogenetics demonstrated a c-statistic greater than 0.6 in the univariate analysis. In the multivariate analysis, variables impacting 1-year OS included hemoglobin (HR 0.69, CI 0.48-0.99, p=0.04), remission status at the time of transplant i.e., not reached (NR) vs complete remission 1 (CR1) (HR 0.05, CI 0.00-0.71, p=0.027), and abnormal cytogenetics (HR 7.55, CI 2.32-24.58, p=0.001). Interestingly, the HCT-CI did not significantly impact 1-year OS in the univariate analysis (c-statistic 0.51, HR 0.99, CI 0.85-1.15, p=0.896). Our prediction model showed good performance with a mean area under the receiver operating characteristic curve of 0.82.
Conclusion: We developed a comprehensive prediction model to assess 1-year OS in elderly patients undergoing alloHCT to overcome some of the limitations of the HCT-CI in this unique age group. This model holds potential to enhance decision-making regarding candidacy for elderly patients undergoing alloHCT.
Disclosures
Farhadfar:Incyte: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Wingard:F2G: Consultancy; Orca: Consultancy; Takeda: Consultancy; Celgene: Consultancy; Cidara: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal