Background: Approximately half of patients with acute graft-versus-host disease (aGVHD) after allogeneic hematopoietic cell transplantation (alloHCT) experience steroid-refractory aGVHD (SR-aGVHD) disease. Patients with SR-aGVHD require intense and prolonged immunosuppression resulting in high risk of severe opportunistic infections and risk of relapse of malignancy which ultimately results in significant increase in short- and long-term morbidity and mortality, poor quality of life for children and their families and increases financial burden on families and health care system.
Corticosteroids have been the mainstay of therapy for new onset acute GVHD. However, treatment for SR-GVHD in children is a serious unmet need. To date, few therapies have been investigated for SR-aGVHD in robust clinical trials for children. Moreover, long-term benefits and survival data from these trials are often lacking or unclear.
A systematic literature review (SLR) was conducted to better understand the efficacy of therapies under investigation in this population.
Methods: Literature database searches of Embase, MEDLINE, Cochrane, and Econlit, were performed on 22 May 2023 according to Cochrane guidelines to identify randomized and non-randomized clinical trials in which the pediatric population with SR-aGVHD represented at least 50% of the trial population. Clinical trials extensions and long-term follow-up reports were also included. Studies published in English from 2009 onwards were retrieved. Additional manual searches of relevant conference abstracts published in the past 3 years were performed.
Results:
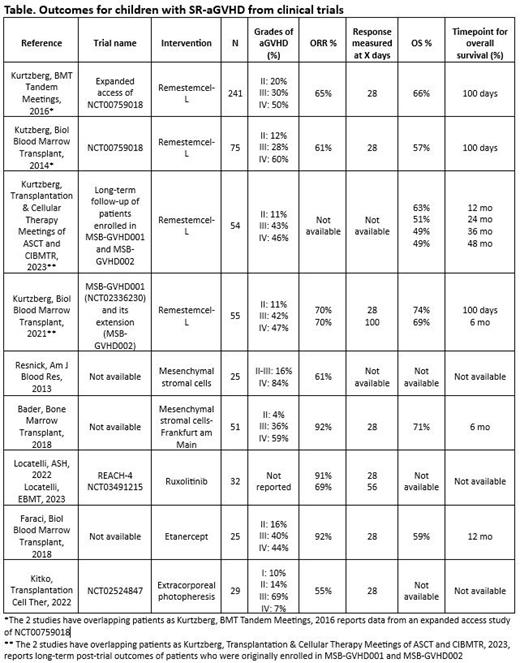
Overall, 29 publications (23 unique studies) met the selection criteria, representing a total of 801 patients aged 18 years or younger with SR-aGVHD. A limited number of interventions were investigated in this population and most studies had a non-randomized design (19 out of 23 studies).
Overall survival (OS) and response rates (ORR) were analyzed from studies enrolling more than 20 pediatric patients with SR-aGVHD (9 studies, Table), which investigated therapies such as mesenchymal stromal cells (MSC), remestemcel-L (MSC manufactured by Mesoblast), ruxolitinib, etanercept and extracorporeal photopheresis. At least 80% of patients treated with cellular therapies (remestemcel-L/MSC) and etanercept had grade III or IV disease.
ORR on day 28 was 92% with etanercept, 77% to 91% with ruxolitinib, 61% to 92% with mesenchymal stromal cells, 61% to 70% with remestemcel-L, and 55% with extracorporeal photopheresis. Durable response rates of 69% at day 56 and 70% at day 100 and were noted for remestemcel-L and ruxolitinib, respectively.
OS at 6 months was 69% with remestemcel-L, and 71% with mesenchymal stromal cells. OS at 1 year was reported for remestemcel-L (63%) and etanercept (59%). Long-term survival data beyond 1 year of patients enrolled in the clinical trial was available only for remestemcel-L and showed an OS rate of 51% at 2 years and 49% at 3 and 4 years. 4-year OS probability for patients with grade III or IV (48% in both cases) was similar to that for the overall population.
Conclusions:
Only a few small size clinical trials were conducted for children with SR-aGVHD which poses a unique challenge for FDA to approve new therapies for SR-aGVHD. Despite a small number (n=9: 5 ruxolitinib arm and 4 control arm) of children (12-18 years) enrolled in REACH II study, ruxolitinib was FDA approved for children>12 years of age.
Long-term efficacy outcomes of children enrolled in clinical trials were reported only for remestemcel-L and showed that approximately half survived beyond 3 years. While other reports showed that grade III/IV disease is associated with poorer outcomes compared to grade I/II, similar survival rates were noted for patients with grade II, III and IV treated with remestemcel-L. In children with SR-aGVHD, other therapies such as ruxolitinib or etanercept demonstrated good response rates, however, the associated survival benefit is unclear.
Disclosures
Satwani:Mesoblast: Consultancy; Sobi: Consultancy. Musat:Cytel, Inc.: Current Employment. Wehling:Mesoblast Ltd.: Current Employment. Shang:Mesoblast Ltd.: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal