Background: Thrombopoietin receptor agonists(TPO-RAs)are effective and safe in immune thrombocytopenia (ITP), and are currently the most important second-line drugs. The effect of TPO-RAs is transient, platelet counts usually return to baseline within 2 weeks unless treatment is continued. Only one-third of patients can maintain after drug discontinuation, but the mechanism is unknown and worthy of further exploration.
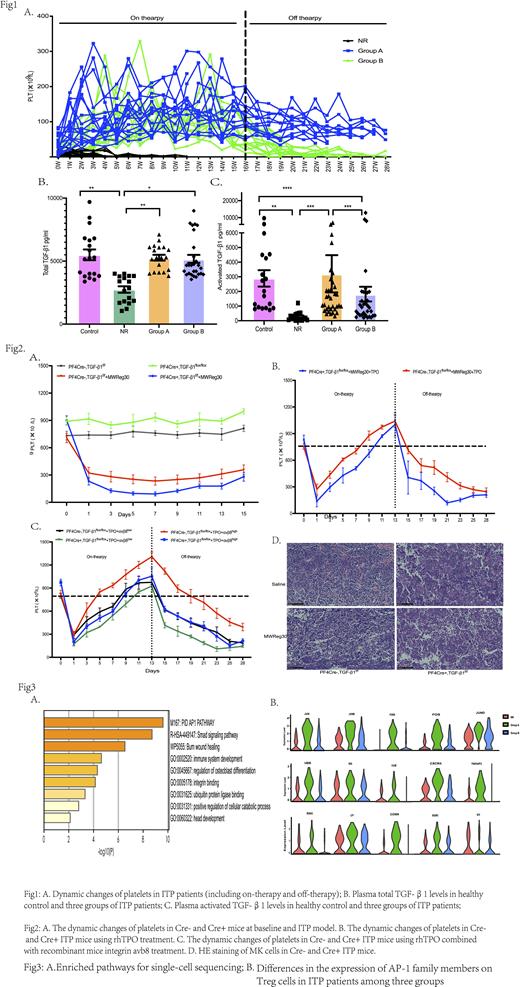
Method and Results:In this study we observed 66 patients treated with TPO-RAs and followed-up to three months after drug withdrawal. Patients were divided into three groups: non-response group (NR), sustained group (Group A), and non- sustained group (Group B) (Fig1-A). The blood samples were collected at the drug discontinuation, and we found that compared with total TGF-β1 levels,plasma activated TGF-β1 levels were extremely lower in NR patients and were also reduced in group B (Fig1-B,C). At the same time, regulatory T cells have the same trend. By using the qPCR and Western blot we preliminary found that integrin αvβ8 regulate the activation of TGF-β1 to promote the formation of Tregs, which affects the sustained response to TPO-RAs withdrawal. Furthermore, lower levels of activated TGF-β1 in the bone marrow were associated with higher numbers of megakaryocytes, fewer plate-producing megakaryocytes, and decreased proportion of hyperploid megakaryocytes (≥16N).
Through in vitro cell culture, we found that recombinant human integrin avb8 protein can promote Treg differentiation and inhibit T cell activation in ITP patients' PBMCs, but this phenomenon can be reversed by anti-β8 antibody. Then, we constructed PF4-TGF-β1 conditional knockout mice (Cre+ mice) and constructed of an ITP passive model by injecting CD41 antibody and found that the Cre+ mice have higher platelets at baseline, while the platelets dropped rapidly than Cre- mice after CD41 antibody injection (Fig2-A). Using rhTPO to treat the ITP mice, we found that Cre+ mice were less effective than Cre- mice, and the sustained response time was shorter after drug withdrawal. When sacrificed the mice, decreased Treg cells and elevated M1 macrophages were detected in Cre+ mice. Meanwhile, HE staining of bone marrow found a greater number of megakaryocytes, but the cell diameter decreased significantly (Fig2-B, D). Next, we treated the mice with rhTPO in combined with recombinant mice integrin avb8 (50ng/kg or 250ng/kg), the therapeutic efficacy and sustained response time in the high-dose avb8 group were significantly improved (Fig2-C)
We also constructed a more representative ITP active model of autoimmune diseases, by immunizing CD61KO mice to produce autoantibodies and infusing spleen cells back into SCID mice. Simultaneously through transfection siRNA or shRNA to activate or silence mouse T cell integrin β8 and reinfuse it into SCID mice. We found that activating integrin β8 in mice had a more significant therapeutic effect and maintained a longer response time.
Finally, through single-cell sequencing analysis, the smads signaling pathways,integrin binding pathways and AP-1 family signaling pathways were significantly enriched in group A patients. Further study confirmed that TPO-RAs can regulate the AP-1 family, which upregulated the β8 subunit, resulting in enhanced activation of TGF-β1 (Fig3-A, B).
Conclusion: Integrin αvβ8 activates TGF-β1 and upregulates Tregs to restore immune intolerance in ITP patients, meanwhile, activated TGF-β1 also promotes hyperploidy megakaryocyte differentiation in bone marrow, which co-regulates the sustained response after TPO-RAs discontinuation.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal