Background: The outcomes of patients with triple and penta-class relapsed refractory multiple myeloma (RRMM) are poor. B-cell maturation antigen (BCMA)-directed chimeric antigen receptor T-Cell (CAR-T) therapies in RRMM have been shown to improve outcomes since approval in 2021; however, relapses are common. We previously reported that patients lost BCMA expression after BCMA-directed therapy. Early re-emergence of surface BCMA on plasma cells (PC) in bone marrow (BM) may be a prognostic indicator for clinical relapse. Furthermore, there is limited published literature about clinical factors that increase risk of early BCMA re-emergence as well as clinical relapse post-CAR-T therapy.
Methods: The study is a prospective cohort study that includes RRMM patients who underwent BCMA CAR-T at the University of Kansas Health System between May 2021 and May 2023 and were followed at regular intervals for the first-year post-infusion. All patients had standard procedure of BM evaluation at 1, 3, and 6 months, and 1 year. Eight-color MFC was performed on the BM specimens. BCMA antibody was purchased from R & D Systems (Minneapolis, MN). Data analysis was performed using FCS Express 5 software (De Novo Software, Los Angeles, CA). 500,000 to up to 2,000,000 events were acquired in all the cases. Responses were evaluated using the International Myeloma Working Group criteria. BCMA re-emergence was defined as BCMA greater than nadir on either polyclonal or monoclonal BM plasma cells.
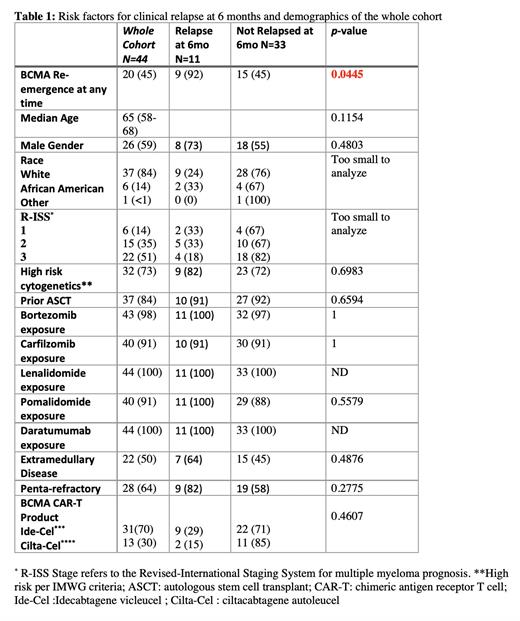
Results: 57 patients were evaluated and 44 patients (77 %) who lost their PC BCMA expression by multicolor flow cytometry (MFC) on bone marrow biopsy (BMBX) at Day 30 from baseline were included in the analysis. Demographic data of all 44 RRMM BCMA CAR-T recipients is shown Table 1. Median follow-up was 7 (4-14) months. Of the 44 patients, BCMA re-emergence was as follows: at 3-months (n=14, 32% of total sample), 6-months (n=5, 11%), 9-months (n=0, 0% of total sample), and 12-months (n=5, 11% of total sample). 14 (32%) of the 44 total patients had clinical relapse within 12 months. Of the 14 patients, 11 (79%) had BCMA re-emergence at any time point. 10 out of 11 (91%) of these patients had BCMA re-emergence before or at the same time as clinical relapse. The median time to BCMA re-emergence was 3 months and the median time to clinical relapse was 6 months. Only 1 patient had clinical relapse prior to BCMA re-emergence.Furthermore, at the 6-month mark, there were no significant clinical or demographic factors between those who relapsed vs. those who had a response to CAR-T therapy other than BCMA re-emergence (p-value 0.0445, Table 1).
Conclusion: BCMA re-emergence on BM detected by MFC may be a prognostic indicator for clinical relapse in RRMM recipients of BCMA CAR-T therapies. It may be valuable to carry out close monitoring that includes evaluation of BCMA expression on BMBx at regular intervals post CAR-T therapy. Additional prospective studies with larger cohorts are needed to determine the risk factors for early BCMA re-emergence and clinical relapse in RRMM patients who receive BCMA CAR-T therapy.
Disclosures
McGuirk:EcoR1 Capital: Consultancy; Magenta Therapeutics: Consultancy; Novartis: Research Funding; Fresenius Biotech: Research Funding; Astellas Pharma: Research Funding; Bellicum Pharmaceuticals: Research Funding; Allovir: Consultancy, Research Funding; Juno Therapeutics: Consultancy; Kite: Consultancy, Research Funding; Gamida Cell: Research Funding; Pluristem Therapeutics: Research Funding. Ahmed:Kite: Consultancy, Research Funding; BMS: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal