CONCLUSIONS
As lenalidomide becomes increasingly established standard of care for patients with newly diagnosed multiple myeloma (NDMM), a significant number of patients may develop resistance to lenalidomide in their treatment, representing a clinically important population in need of effective therapies. Lenalidomide-refractory patients, who relapse quickly and have very poor outcomes, constitute a population with unmet medical needs. Selinexor is a selective inhibitor of exportin-1, leading to the accumulation of tumor suppressor proteins and growth regulator proteins in the nucleus. This results in reduced expression of several oncoproteins, leading to cell cycle arrest and apoptosis of cancer cells. Previous data indicate that the biology associated with resistance to the monoclonal antibody daratumumab is linked to sensitivity to selinexor (ASH 2021), and selinexor conbombined with daratumumab and dexamethasone (SDd) is a well-tolerated and effective option for patients with relapsed/refractory multiple myeloma (RRMM) (Gasparetto C, 2020). Here, we present the safety and preliminary efficacy findings from the first study of SDd combination therapy in Chinese patients with multiple myeloma who refractory to lenalidomide at first relapse (NCT05559788).
This study is a single-arm, prospective, non-interventional, real-world study that enrolled pts who had previously received one-line anti-myeloma treatment. Subjects were treated with selinexor (60 mg QW) in combination with daratumumab (16 mg/kg per label) and dexamethasone (40 mg QW) in 28-day cycles until PD, death or patients' withdrawal. Data collected included treatment response, patient characteristics such as age, sex, multiple myeloma features (cytogenetics by fluorescence in situ hybridization, type, stage, anti-myeloma treatment, and stem cell transplantation [SCT] status), and patients' survival.
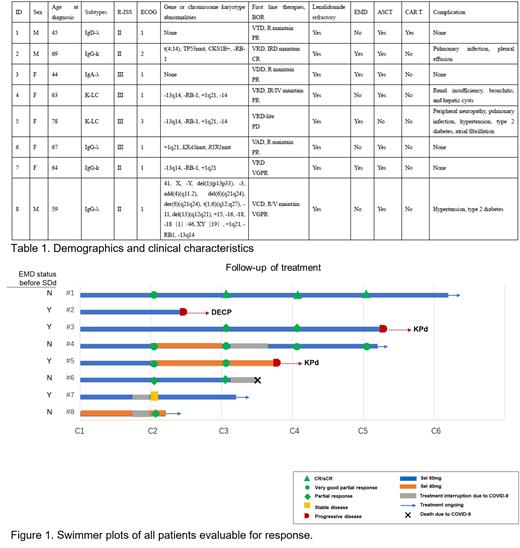
Between Feb 1, 2023, and May 30, 2023, a total of 8 RRMM pts were enrolled and received treatment at The First Affiliated Hospital of Soochow University. The median age was 63 years (range 44-78), with all pts (100%) had R-ISS stage II/III disease. Among them, 6 (75%) pts were classified as high risk or above by mSMART risk stratification, and 4 (50%) pts had extramedullary disease (EMD). All 8 (100%) pts were refractory to lenalidomide, and 7(87.5%)have received autologous stem cell transplantation (ASCT) (Table 1). At the end of one course, 6 patients (75%) achieved remission (3 very good partial responses [VGPR] and 3 partial responses [PR]). Among the 4 pts with EMD, 1 pt showed progressive disease (PD) after two courses, 1 pt showed stable disease (SD), and 2 pt achieved PR and VGPR but progressed after four and two courses, respectively. The data suggests that SDd combination therapy brings about rapid remission in EMD patients, while the duration of response may be shorter. Therefore, for these patients, it is crucial to closely monitor and plan for the next steps of treatment after achieving rapid remission. All four remaining non-EMD pts achieved remission, three of whom are continuing treatment (one died of COVID-19).
Four patients were infected with COVID-19 during the treatment process. One pt was asymptomatic and detected positive with virus infection by antigen test screening. Among the three pts with symptoms, one had fever, cough, and pneumonia, and one had multiple organ failure (MOF). Three pts received antiviral medication (two with Paxlovid and one with Molnupiravir), and three recovered, but the one with MOF died. The SDd treatment was interrupted in all four pts, with a median time of 18 days (range: 12 to 21 days), until COVID-19 turned negative. The most common grade 3 adverse events (AEs) were thrombocytopenia (25%), anemia (13%), and neutropenia (13%). Non-hematological AEs were mainly grade 1-2. Three patients had dose reductions due to myelosuppression.
This real-world study demonstrates that SDd combination therapy led to early, deep responses in lenalidomide-refractory multiple myeloma patients at their first relapse. Considering that patients with extramedullary disease had limited benefit from SDd treatment, future study designs may restrict the recruitment of such patients.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal