Introduction: Findings from a previously conducted matching-adjusted indirect comparison (MAIC) between isatuximab plus carfilzomib with dexamethasone (IsaKd) and daratumumab plus lenalidomide and dexamethasone (DRd) among relapsed and/or refractory multiple myeloma (RRMM) patients receiving one to three prior lines of therapy (LoT) demonstrated a significantly better progression-free survival (PFS) and a positive trend for overall survival (OS) in favor of IsaKd compared with DRd (median follow up of 20.7 months for IKEMA trial). Additionally, IsaKd demonstrated an acceptable safety profile with significantly lower rates of several treatment-emergent adverse events than DRd [Richter J et al, Cancer Med 2023;12(7):8005-8017; Richter J et al.(Publication Number 1962), 63 rd ASH Annual Meeting and Exposition, 2021; Richter J, et al. P-213, Clin Lymphoma Myeloma Leuk. 2021;21(Suppl 2):S156-S157]. The present study used longer follow-up data from the phase 3 IKEMA trial (NCT03275285) to update the previous MAIC evaluating the comparative efficacy of IsaKd versus DRd.
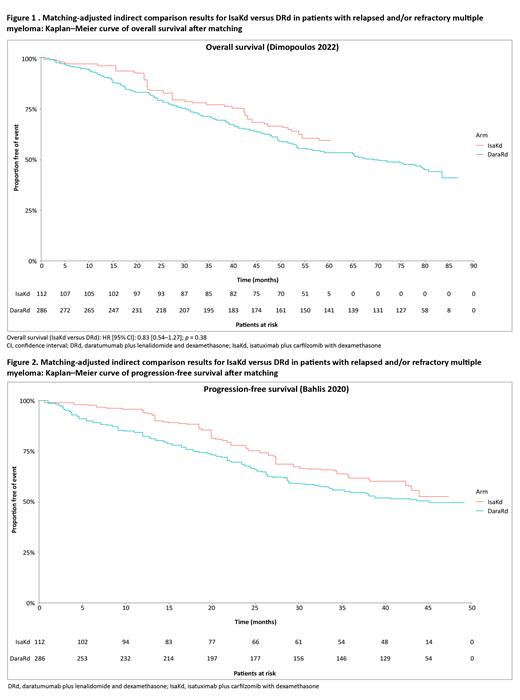
Methods: A MAIC was performed with the latest available individual patient-level data (IPD) from the IKEMA trial (IsaKd arm, n = 179) (data cutoff: February 2023 for OS and January 2022 for PFS), and aggregate data from study publications of phase 3 POLLUX trial (DRd arm, n = 286, NCT02076009) [Bahlis NJ et al. Leukemia 2020;34(7):1875-1884; Dimopoulos MA et al. Hemasphere 2022;6:13]. Patients were selected from the IsaKd arm of the IKEMA trial to match the inclusion and exclusion criteria of the POLLUX trial. Individual patients from the IKEMA trial were then assigned weights such that (1) weighted mean baseline characteristics for patients in the IsaKd arm matched those reported for patients in the DRd arm, and (2) each patient's weight was equal to their estimated odds (propensity) of being in the POLLUX trial versus the IKEMA trial. After matching, outcomes were compared across the two trial populations. For PFS and OS, hazard ratio (HR) and 95% confidence interval (CI) were reported from a Cox proportional hazards (PH) model using the weighted sample for IsaKd and the pseudo-IPD for DRd extracted from published Kaplan-Meier curves via digitization software [Mark M et al, Engauge Digitizer Software; http://markummitchell.github.io/engauge-digitizer]. For any violations of the PH assumption, the restricted mean survival time (RMST) at median follow-up of IKEMA for the outcome of interest was calculated instead.
Results: After the MAIC adjustment, all available baseline characteristics were well balanced across the trial populations. For PFS, as the PH assumption was violated ( χ2 = 5.52, p = 0.019), RMST method was used to compare the two treatments. PFS was significantly better with IsaKd versus DRd (mean difference [95% CI]: 3.63 months [0.14-7.12]; p = 0.04) at 44 months follow-up using RMST method. For OS, the PH assumption ( χ2 = 0.89, p =0.35) was not violated. A numeric, statistically non-significant trend in favor of IsaKd versus DRd was observed for OS (Figure 1).
Conclusions: This MAIC analysis demonstrated a significant benefit in PFS and a positive trend in the improvement of OS with IsaKd compared with that with DRd. These findings with a longer follow-up were consistent with the previous analyses conducted with a follow-up of 2 years. These data highlight the therapeutic potential of IsaKd, a lenalidomide-free regimen, in RRMM patients receiving one to three prior LoT compared with a lenalidomide-based regimen.
Disclosures
Richter:Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Genentech: Consultancy; Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; Bristol-Meyers-Squibb: Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy; Sanofi: Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Consultancy. Lin:Sanofi: Current Employment, Current holder of stock options in a privately-held company. Garcia-Horton:Sanofi: Research Funding; Analysis Group: Current Employment. Guyot:Sanofi: Current Employment, Current holder of stock options in a privately-held company. Zhou:Analysis Group: Current Employment; Sanofi: Research Funding. Sievert:Sanofi: Current Employment, Current holder of stock options in a privately-held company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal