Background: CLL is a rare, incurable, relapsing disease occurring in approximately 0.3/100,000 individuals in China. Acalabrutinib (acala) is a highly selective Bruton tyrosine kinase inhibitor with minimal off-target activity approved in the US and Europe for treatment of CLL. Pivotal studies supporting acala's approval had limited inclusion of Asian populations. In the first phase 1/2 trial (NCT03932331) in Chinese pts with R/R CLL, acala demonstrated high response rates and acceptable tolerability at the 6-mo follow-up (Yang S et al. HemaSphere, 2023;7(S3):3716). We present 12-mo results from this trial.
Methods: Adults with active CLL, Eastern Cooperative Oncology Group (ECOG) performance status ≤2, and who received ≥1 prior systemic therapies for CLL were enrolled to receive acala 100 mg twice daily in 28-day cycles until disease progression or treatment discontinuation due to adverse events (AEs) presenting substantial clinical risk. The primary endpoint was overall response rate (ORR) per 2018 International Workshop on Chronic Lymphocytic Leukemia criteria as assessed by blinded independent central review (BICR). Secondary endpoints included duration of response (DOR) and progression-free survival (PFS) as assessed by BICR; ORR, DOR, and PFS as assessed by investigators (INV); overall survival (OS); and AEs.
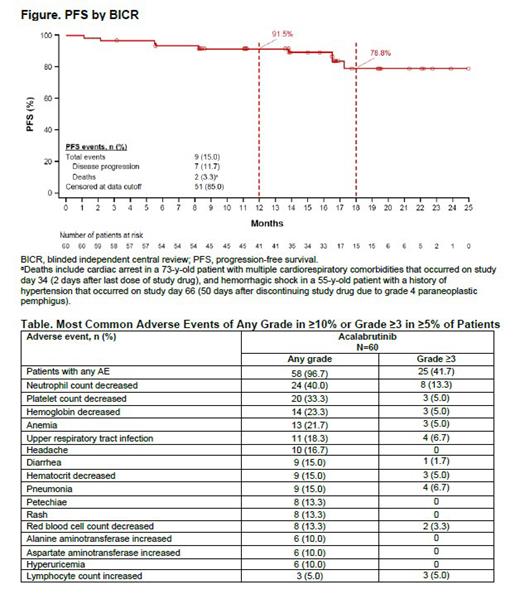
Results: Sixty pts from 20 sites in China received acala (median age 62 y; 31.7% female; 21.7% with del(17p) and/or TP53 mutation; 51.7% with unmutated IGHV; median 1 prior line of therapy). At the updated data cutoff of December 22, 2022, 53 pts (88.3%) were receiving acala; reasons for acala discontinuation were progressive disease (5.0%; n=3), AEs (3.3%; n=2), pt decision (1.7%; n=1), and other (1.7%; n=1). Median duration of treatment was 19.4 mo (range 0.6-28.2). BICR-assessed ORR was 85.0% (95% CI 73.4, 92.9); no pt had complete response (CR). Median DOR by BICR was not reached (NR). At a median follow-up of 20.2 mo, median PFS by BICR was NR; the estimated 12-mo and 18-mo PFS rates were 91.5% (95% CI 80.9, 96.4) and 78.8% (95% CI 60.9, 89.2), respectively ( Figure). When assessed by INV, ORR was 85.0% (95% CI 73.4, 92.9) with 1 pt having CR, median DOR was NR, and median PFS was NR; estimated 12-mo and 18-mo PFS rates were 95.0% (95% CI 85.2, 98.3) and 87.2% (95% CI 69.1, 95.0), respectively. Median OS was also NR; the estimated 12-mo and 18-mo OS rates were both 96.7% (95% CI 87.3, 99.2). AEs of any grade were reported in 58 pts (96.7%); 25 pts (41.7%) had grade ≥3 AEs, most commonly decreased neutrophil count (13.3%, n=8), pneumonia, and upper respiratory tract infection (each 6.7%, n=4) ( Table). AEs leading to treatment discontinuation included paraneoplastic pemphigus (n=1) and rectal neoplasm (n=1). AEs leading to dose interruption were reported in 13 pts (21.7%), most commonly COVID-19 (5.0%), neutrophil count decreased (3.3%), and platelet count decreased (3.3%). Nine pts (15.0%) had serious AEs (SAEs); only 1 SAE occurred in >1 pt (pneumonia, n=2). Fatal AEs (occurring ≤30 d after last dose) were reported in 1 pt (cardiac arrest in a 73-y-old pt with multiple cardiorespiratory comorbidities on study day 34). Among events of clinical interest, neutropenia was reported in 24 pts (40.0%) and hypertension was reported in 3 pts (5.0%). There were no cases of atrial fibrillation/flutter, major hemorrhage, interstitial lung disease/pneumonitis, second primary malignancies, or tumor lysis syndrome.
Conclusions: Twelve-mo data in Chinese pts with R/R CLL treated with acala demonstrated a high ORR, with responses that were durable and clinically meaningful. Continuous treatment with acala was well tolerated with manageable toxicities; no unexpected safety observations were observed in Chinese pts with R/R CLL.
Disclosures
Li:AstraZeneca: Current Employment. Butturini:AstraZeneca: Current Employment, Current equity holder in private company. Wang:AstraZeneca: Current Employment. Liu:AstraZeneca: Current Employment. Lai:AstraZeneca: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal