INTRODUCTION
Therapeutic options for Chronic Lymphocytic Leukemia (CLL) have advanced significantly over time, resulting in a heterogeneous treatment landscape. Insights into the depth of treatment response can be gained through highly-sensitive detection of residual cancer cells using measurable (also known as minimal) residual disease (MRD) testing. Initially, MRD testing was limited to prognostic use within clinical trials. However, in recent years it has expanded into various clinical settings, although the extent of real-world use is not well established.
METHODS
766 individuals with CLL enrolled in the Leukemia and Lymphoma Society (LLS) registry had their medical records collected and data extracted via the Invitae Ciitizen® platform, a patient-centric platform that leverages HIPAA right-of-access to organize clinical data into a standardized, research-ready format, using a combination of machine learning and human clinician review. Proprietary logic-based algorithms were used to characterize lines of therapy (LOTs), MRD testing methods, outcomes and duration of therapy. Descriptive findings are reported below.
RESULTS
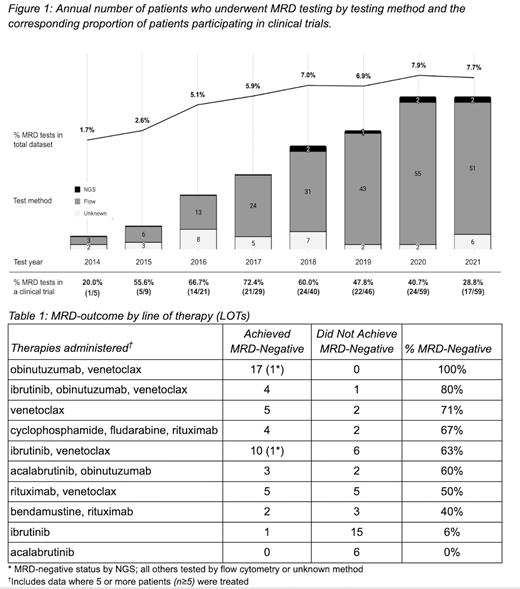
Of the 766 CLL participants, 117 (15%) received a total of 311 MRD tests (mean=3 tests/person, median=2, max=16). 67 individuals had one or more MRD negative results (134 negative results total) and 78 individuals had one or more MRD positive results (177 positive results total). 56/117 (47.9%) patients had one or more MRD tests as part of a clinical trial, for a total of 132/311 (42.2%) MRD tests performed during a trial. Total MRD tests included 260 (83.6%) evaluated by flow cytometry, 8 (2.6%) by next generation sequencing (NGS), with the remainder not having documented methods (43; 13.8%).
Since 2014, when MRD testing was integrated as a prognostic indicator in clinical trials, there has been increasing use of MRD testing ( Figure 1) within this cohort, as assessed by percentage of individuals receiving one or more tests per year. Interestingly, there is a peak of percentage tested in a clinical trial setting in 2017, after which a continued increase in those tested outside of a clinical trial setting shifts the overall proportion down. We also note the appearance of NGS testing in 2018.
The 117 individuals who were MRD tested received 40 unique LOTs (e.g., “obinutuzumab, venetoclax”). We then quantified the MRD outcomes as either “achieved MRD-negative” or “did not achieve MRD-negative” for the most commonly-used therapies (n≥5) ( Table 1). Ibrutinib and acalabrutinib monotherapies had the lowest proportion of MRD-negative outcomes (1/15 and 0/6, respectively), whereas obinutuzumab-venetoclax +/- ibrutinib combination therapies had the highest proportion of MRD-negative outcomes (4/5 and 17/17, respectively).
In order to assess the impact of MRD outcome on treatment patterns, the duration of therapy following a MRD-positive and negative test were evaluated. Therapy continued a mean duration of 691 days (n=126, median=488) after a MRD-positive test and 419 days (n=66, median=230) after a MRD negative test.
CONCLUSION
In this real-world dataset of 766 individuals with CLL, we detect an increasing rate of MRD testing since 2014. Notably, this usage is not limited to the clinical trial setting, underscoring the growing significance of MRD testing as a more general tool for evaluating disease control. Exploration of which LOTs achieve proportionally more MRD-negative statuses highlights that venetoclax-containing fixed-duration regimens as well as venetoclax monotherapy have higher rates of MRD-negativity. Conversely, BTK monotherapies ibrutinib and acalabrutinib were associated with the lowest percentage of MRD-negative outcomes. Finally, we find that the duration of therapy from the time of MRD testing is shorter when the result is MRD-negative, suggesting that MRD testing as a component of outcomes assessment used in the real-world setting may be useful in deciding when to discontinue therapy. This aligns with ongoing clinical trials investigating fixed duration therapies and the use of MRD-negative status as an outcome measure to assess depth of remission. Future studies on larger datasets will explore how MRD testing is integrated into clinical practice and its impact on treatment decisions.
Disclosures
Bacchus, MHS, PA-C:Bristol Myers Squibb: Current equity holder in publicly-traded company; Syndax: Current equity holder in publicly-traded company; Invitae: Current Employment, Current equity holder in publicly-traded company. Singh-Bulkan:Invitae: Current Employment, Current equity holder in publicly-traded company. Littleford:Invitae: Current Employment, Current equity holder in publicly-traded company. Kim:Invitae: Current Employment, Current equity holder in publicly-traded company. Martin:Tango Therapeutics: Current equity holder in publicly-traded company; ProKidney: Current equity holder in publicly-traded company; Pfizer: Current equity holder in publicly-traded company; IBM: Patents & Royalties; Invitae: Current Employment, Current equity holder in publicly-traded company. Lacoste:Invitae: Current Employment, Current equity holder in publicly-traded company. Brimble:Invitae: Current Employment, Current equity holder in publicly-traded company. O'Brien:Invitae: Current Employment, Current equity holder in publicly-traded company. Nottke:Invitae: Current Employment, Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal