Background: Histiocytic neoplasms represent a diverse group of clonal hematopoietic disorders, varying in severity from xanthogranuloma to multisystemic Erdheim-Chester Disease (ECD). Both Langerhans cell histiocytosis (LCH) and non-Langerhans cell histiocytosis (nLCH) are characterized by activating mutations in the MAPK pathway, primarily involving BRAF point mutations. In this report, we present a compelling case of disseminated xanthogranuloma with a unique BRAF fusion, which exhibited a remarkable therapeutic response to MEK inhibition. This finding adds to our understanding of the molecular heterogeneity within histiocytic neoplasms and highlights the potential significance of targeted therapies in managing these rare but clinically significant disorders
Clinical Observation: In this clinical observation, we present the case of a 71-year-old female who sought medical attention due to a 3-year history of progressive cutaneous lesions, accompanied by hoarseness and blurry vision. A biopsy of a lesion revealed characteristic features consistent with xanthogranuloma. Further examination through direct laryngoscopy unveiled lesions on the vocal cords and tracheal masses, which exhibited identical histologic findings as the skin biopsies.
A comprehensive analysis involving massively parallel DNA sequencing revealed the presence of a GAB2-BRAF fusion, along with a tumor mutational burden of 1 mutation/megabase (Mb). Immunohistochemical testing of the endobronchial biopsy demonstrated a combined positive score of 10% for PDL-1 expression, suggesting the potential for immune checkpoint blockade.
Given the implicated role of MAPK pathway activation due to the identified fusion, a therapeutic approach utilizing the MEK inhibitor Trametinib was initiated. Encouragingly, the patient exhibited a favorable response with a reduction in tumor burden, near-resolution of hoarseness, and improved vision. However, this treatment was accompanied by MEK inhibitor-associated cardiotoxicity, leading to the discontinuation of Trametinib.
Regrettably, following the cessation of Trametinib, the patient's lesions rapidly progressed, and her disease worsened beyond the pre-treatment state. Taking into account the 10% PDL-1 positivity, immunotherapy with Pembrolizumab was commenced, resulting in partial improvement. Nonetheless, the patient developed immunotherapy-related cardiotoxicity.
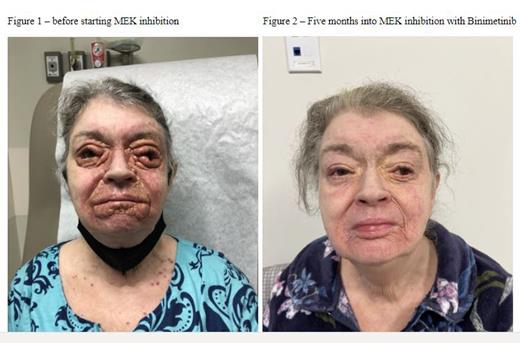
In light of the continued disease progression off therapy, the MEK inhibitor Cobimetinib was introduced. The patient responded rapidly to this therapeutic intervention; however, during a one-week break in Cobimetinib treatment, the disease worsened back to the baseline. Consequently, Cobimetinib was discontinued, and a continuously dosed MEK inhibitor, Binimetinib, was cautiously administered at a lower dose to mitigate the risk of cardiotoxicity.
As of the time of this report, the patient remains on Binimetinib, achieving complete clinical remission, and there is no evidence of further cardiotoxicity. This clinical observation underscores the challenges and complexities in managing advanced xanthogranuloma with unique molecular alterations and highlights the importance of closely monitoring and tailoring therapies to optimize patient outcomes.
Conclusion: To our knowledge, this is the first clinical trial documenting MEK inhibition for a patient with xanthoma disseminatum harboring a BRAF fusion. Seeking to target downstream of BRAF, MEK inhibition demonstrated a dramatic response, with rapid relapse upon cessation, supporting a central oncogenic role for BRAF fusion. Further studies should explore alterations in the RAS-BRAF-ERK pathway in nLCH and consider clinical trials with MEK inhibitors. Our clinical experience suggests continuous MEK inhibitor exposure may be necessary for effective disease control. These findings advance our understanding of therapeutic options in histiocytic neoplasms, paving the way for more precise and impactful treatments.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal