Background
Tyrosine kinase inhibitors (TKIs) have significantly improved the life expectancy of individuals with chronic myeloid leukemia (CML), bringing it closer to that of the age-matched general population 1. However, clinical trial data from the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) Program indicates that only 3.8% of CML patients aged 75 or above participate in trials, despite this age group representing about 30% of CML patients 2. This study aims to assess the outcomes of elderly patients aged 75 years or older diagnosed with CML.
Methods
We conducted a retrospective study, using electronic medical records of consecutive CML patients aged ≥ 75 years, diagnosed between January 2002 and December 2021 at four hematological centers in Israel and Moffitt Cancer Center in Florida, United States (MCC). One-year and five-year overall survival (OS) rates were calculated for the whole cohort and for octogenarians (above 80 years). In addition, to assess if CML diagnosis affected life expectancy, we estimated the expected OS of the Israeli cohort based on life expectancy data from the central bureau of statistics (CBS). Kaplan-Meier curves were used to compare expected and observed median OS with the Log-rank analysis. Local Institutional Review Boards approved the study.
Results
A total of 123 patients (78 treated in MCC and 45 in Israel) aged ≥ 75 years were diagnosed with CML, with a median age of 79 (range: 75 - 100) years, and 55 patients (45%) were octogenarians. At the time of CML diagnosis 84% of had comorbidities, including cardiovascular risk factors in 90 patients (73%), while 50 patients (41%) had cardiovascular/cerebrovascular diseases. Most patients (93%) were diagnosed in chronic phase CML and high/intermediate EUTOS-LTS risk score (96%) treated with imatinib in the 1 st line treatment (69%). After a median duration of 15 (range:1-153) months on 1 st line treatment, 71 patients (58%) discontinued therapy primarily due to intolerance (n=51) while other causes included resistance (n=15), noncompliance/insurance issues (n=4) or progression to blast crisis (n=1). The 2 nd -line treatment included mainly 2 nd generation TKIs (n=53, 74%) (dasatinib-26, nilotinib -19 and bosutinib-8), while 9 patients received imatinib, 1 patient received nilotinib and imatinib, 5 patients received hydroxyurea and 3 patients were lost to follow-up. 35 patients (28%) reached 3 rd line of treatment (imatinib- 5, dasatinib- 3, bosutinib-12, nilotinib-5, ponatinib-2, asciminib-1, hydroxyurea -1, loss to follow up- 6) chiefly (28 patients) due to intolerance and others (7 patients) due to resistance.
The best response assessed by RQ-PCR showed deep molecular response (DMR) in 50%, major molecular response (MMR) in 16%, and complete cytogenetic response (CCyR) in 11% of patients with a median time to maximal response of 19 (range: 0.5-111) months. Nevertheless, treatment-free remission (TFR) was rare (n=1). During a median follow-up of 45 (0.4-198) months, 55 (45%) patients died, with cardiovascular complications (n=9), disease progression (n=8), infection (n=7), and secondary malignancy (n=4) being the main known causes.
The median OS for the whole cohort was 72.4 (53.1-91.7) months. Improved OS was documented in patients with an age adjusted charlson comorbidity index< 5 (vs. ≥5, p=0.007), those who achieved DMR (vs. no DMR, p=0.001) and median time to best response of 0-18 months (vs. ≥18, p=0.004). Moreover, OS was improved in patients who received 2 nd generation TKIs in 1 st line treatment, 91.1 (81.5-131) vs. 57.7 (35.4-79.9) months in those who received imatinib, (p=0.023). Older age did not affect OS; 1 and 5-year OS for the whole cohort was 86% and 29%, respectively, and 84.3% and 19.6% for the octogenarians, respectively (p=0.076).
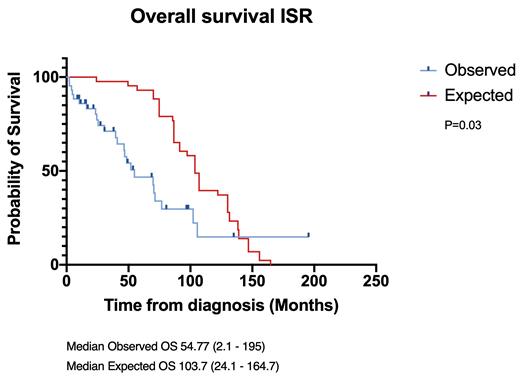
In the Israeli cohort, while the median expected OS was 103.7 (24-164.7) months, the median observed OS was only 54.77 (2.1-195) months (p=0.03).
Conclusions
Elderly CML patients were often diagnosed in chronic phase with high/intermediate risk scores. The majority received imatinib as 1 st line treatment and achieved CCyR and MMR, with half of patients achieving also DMR. Surprisingly, patients treated with imatinib had worse OS compared to those receiving 2nd generation TKIs as 1st line treatment. Furthermore, the Israeli cohort analysis supports reduced life expectancy in the very elderly patients with CML.
Disclosures
Shacham Abulafia:Pfizer: Consultancy; Novartis: Consultancy. Koren-Michowitz:Novartis: Honoraria; Pfizer: Honoraria. Raanani:Janssen: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Consultancy, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal