Introduction
Brentuximab vedotin (BV) with AVD is approved in classical Hodgkin lymphoma (cHL) and has demonstrated statistically significant improvement in 6-year overall survival (OS) as compared with ABVD; associated neuropathy and neutropenia may be related to overlapping mechanisms of BV and vinblastine (Ansell 2022). A study in nonbulky early-stage cHL showed preserved efficacy with improved safety when omitting vinblastine in the BV-AD regimen (Abramson 2023). Here, we present results of a phase 2 trial adding nivolumab to the BV-AD regimen without radiation in patients (pts) with nonbulky early-stage cHL (SGN35-027 Part C).
Methods
SGN35-027 (NCT03646123) Part C enrolled pts with Ann Arbor stage I or II cHL without bulky disease, defined as those with a single node or nodal mass with a <10-cm diameter on computed tomography imaging. Pts received 4 cycles of AN+AD (BV 1.2 mg/kg [A], nivolumab 240 mg [N], doxorubicin 25 mg/m 2 [A], and dacarbazine 375 mg/m 2 [D] intravenously on days 1 and 15 of each 28-day cycle). Per protocol, G-CSF prophylaxis was not required for subjects receiving the treatment regimen. The primary endpoint is complete response (CR) rate at end of treatment (EOT). Secondary endpoints include progression-free survival (PFS), overall response rate (ORR), duration of response (DOR), and duration of complete response (DOCR), as well as safety and tolerability. The Lugano Classification Revised Staging System for malignant lymphoma (Cheson 2014) incorporating Lymphoma Response to Immunomodulatory Therapy Criteria (LYRIC) for nodal non-Hodgkin and Hodgkin Lymphomas (Cheson 2016) per investigator was used to assess disease response and progression. Exploratory endpoints include baseline and longitudinal circulating tumor DNA analysis on a subset of pts.
Results
Part C has completed enrollment with 154 pts having received at least 1 dose of study treatment. The majority of treated pts were white (84%), aged <65 years (92%), and female (55%), and presented with nonbulky stage I (11%) or II (89%) cHL. Median age was 31.0 years (range, 18-77). All findings were based on a data cutoff of 22 May 2023.
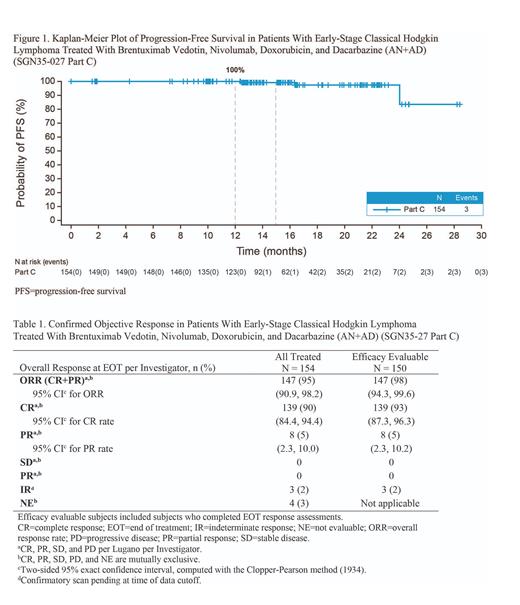
Among 150 efficacy evaluable pts, the ORR was 98% (95% CI, 94.3-99.6) and the CR rate was 93% (95% CI, 87.3-96.3) at EOT (Table 1). Among all pts treated (N = 154), the ORR was 95% (95% CI, 90.9-98.2) and the CR rate was 90% (95% CI, 84.4-94.4) at EOT.
With a median follow-up of 15.2 months, 3 of 154 pts (2%) had progressive disease (at 12.5, 16.4, and 24.0 months) and no deaths occurred. The PFS rate at 12 months was 100% (95% CI, 100-100) (Figure 1). A total of 99.2% of pts (95% CI, 94.3-99.9) had a DOR of least 12 months, and 97.2% (95% CI, 91.5-99.1) had a DOCR of at least 12 months.
The planned treatment course of 4 cycles was completed by 94% of pts. Forty-four percent received G-CSF, primarily for primary prophylaxis, for 1 or more cycles. Three percent of pts discontinued treatment (all drugs) due to treatment-emergent adverse events (TEAEs). No events of febrile neutropenia were reported. Thirty four percent of pts experienced grade ≥3 treatment-related TEAEs, and 3% experienced grade ≥3 treatment-related peripheral sensory neuropathy. The most common grade ≥3 treatment-related TEAEs were neutropenia (9%), increased alanine aminotransferase (ALT) (7%), and increased aspartate aminotransferase (5%). Treatment-emergent immune-mediated adverse events (IMAEs) occurred in 22% of pts, and grade ≥3 treatment-emergent IMAEs occurred in 7% of pts. The most common treatment-emergent IMAE of any grade was hypothyroidism (6%). Twelve percent of pts experienced treatment-related serious TEAEs. The most common treatment-related serious TEAEs were pyrexia (3%), increased ALT (1%), and peripheral sensory neuropathy (1%).
Conclusions
Results from SGN35-027 Part C show promising efficacy and an acceptable safety profile for BV and nivolumab in combination with chemotherapy (AN+AD) for pts with nonbulky early-stage cHL. Pts had high ORR (98%) and CR rates (93%) at EOT, and a 12-month PFS of 100% with a median follow-up of 15.2 months. There was a low incidence of grade 3 or higher neuropathy and there were no cases of febrile neutropenia. AN+AD demonstrates encouraging efficacy in nonbulky early-stage cHL and may avoid toxicities associated with radiation, bleomycin, and vinblastine. Follow-up of Part C is ongoing with 92% of pts in long-term follow-up.
OffLabel Disclosure:
Abramson:Takeda: Consultancy; Celgene: Consultancy; Mustang Bio: Consultancy, Research Funding; MorphoSys: Consultancy; Merck: Research Funding; Lilly: Consultancy; Kymera: Consultancy; Kite Pharma: Consultancy; Janssen: Consultancy, Honoraria; Interius: Consultancy; Incyte: Consultancy; Genmab: Consultancy; Genentech: Consultancy; Epizyme: Consultancy; Century Therapeutics: Consultancy; Cellectar Biosciences: Consultancy; Caribou Biosciences: Consultancy; BMS: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy; AstraZeneca: Consultancy, Honoraria; AbbVie: Consultancy; Ono Pharma: Consultancy; Regeneron: Consultancy, Honoraria; Seagen Inc.: Research Funding; Novartis: Consultancy; EMD Serono: Consultancy; Alimera Sciences: Consultancy; Karyopharm Therapeutics: Consultancy; C4 Therapeutics: Consultancy; Bluebird Bio: Consultancy; AI Therapeutics: Research Funding. Bartlett:Merck: Research Funding; Autolus: Research Funding; Millennium: Research Funding; Bristol Myers Squibb/Celgene: Research Funding; Seagen Inc.: Membership on an entity's Board of Directors or advisory committees, Research Funding; Forty Seven: Research Funding; Janssen: Research Funding; Gilead/Kite: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Research Funding; Roche/Genentech: Membership on an entity's Board of Directors or advisory committees, Research Funding; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding. Burke:Bayer HealthCare Pharmaceuticals: Consultancy; MorphoSys AG: Consultancy; Nurix: Consultancy; Roche/Genentech: Consultancy; Seagen Inc.: Consultancy, Speakers Bureau; Verastem: Consultancy; Morphosys: Research Funding; Gilead Sciences: Consultancy; X4 Pharmaceuticals: Consultancy; Epizyme: Consultancy; Kura Oncology: Consultancy; Kymera: Consultancy; Bristol Myers Squibb: Consultancy; BeiGene: Consultancy, Speakers Bureau; AstraZeneca: Consultancy; Adaptive Biotechnologies: Consultancy; AbbVie: Consultancy. Lynch:SeaGen: Consultancy; Merck: Research Funding; Foresight Diagnostics: Consultancy; Genentech: Research Funding; Rapt: Research Funding; Cancer Study Group: Consultancy; Seagen Inc.: Research Funding; Abbvie: Consultancy; Bayer: Research Funding; Cyteir: Research Funding; Incyte: Research Funding; TG Therapeutics: Research Funding. Domingo Domenech:BeiGene: Consultancy; Takeda: Consultancy, Honoraria, Speakers Bureau. Hess:ADC Therapeutics: Consultancy; Bristol Myers Squibb: Consultancy. Schuster:Pharmacyclics: Speakers Bureau; Genentech: Speakers Bureau. Linhares:Kyowa: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Seagen Inc.: Membership on an entity's Board of Directors or advisory committees, Research Funding; Kyowa Kirin: Speakers Bureau; Curio Science Workshop: Other: participation and moderation; Janssen: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Alexion: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; BeiGene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees. Ramchandren:Bristol Myers Squibb: Consultancy; Merck: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; Seagen Inc.: Consultancy, Research Funding; Curis: Research Funding; Trillium: Research Funding; Cellectar: Research Funding. Gandhi:Janssen Oncology: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees. Shah:AbbVie: Membership on an entity's Board of Directors or advisory committees; Epizyme: Research Funding; Seagen Inc.: Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Research Funding; BeiGene: Research Funding; ADCT: Research Funding. Jurczak:BeiGene: Research Funding; Pfizer: Research Funding; AbbVie: Consultancy; Celgene: Research Funding; SOBI: Research Funding; Roche: Research Funding; Janssen: Research Funding; Eli Lilly: Research Funding; Merck: Research Funding; Bayer: Research Funding; AstraZeneca: Research Funding; Takeda: Consultancy; AbbVie: Research Funding; Roche: Consultancy; SOBI: Consultancy; Eli Lilly: Consultancy; Pfizer: Consultancy; BeiGene: Consultancy; AstraZeneca: Consultancy; Takeda: Research Funding. Re:Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Membership on an entity's Board of Directors or advisory committees; Italfarmaco: Membership on an entity's Board of Directors or advisory committees. Prince:Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Guo:Seagen Inc.: Current Employment, Current equity holder in publicly-traded company. Ho:Seagen Inc.: Current Employment, Current equity holder in publicly-traded company, Other: Travel expenses. Beck:Seagen Inc.: Current Employment, Current equity holder in publicly-traded company. Yasenchak:Seagen Inc.: Consultancy, Research Funding; BeiGene: Speakers Bureau. Lee:Celgene: Research Funding; Century Therapeutics: Consultancy; Cancer Experts: Honoraria; Aptitude Health: Honoraria; Bristol-Myers Squibb: Research Funding; Deloitte: Honoraria; Guidepoint: Honoraria; Janssen: Honoraria; Curio Sciences: Honoraria; Oncternal Therapeutics: Research Funding; Olson Research: Honoraria; Pharmacyclics: Research Funding; Korean Society of Cardiology: Honoraria; Seagen Inc.: Research Funding; Takeda: Research Funding.
Brentuximab vedotin is a CD30-directed antibody-drug conjugate that is FDA-approved for frontline treatment of advanced cHL in combination with AVD. The SGN35-027 Part C study evaluates the efficacy and safety of AN+AD for the treatment of early-stage cHL.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal