Introduction: The prognosis for highly heterogeneous pediatric acute myeloid leukemia (AML), classified into eight major subtypes, varies considerably. Common pediatric AML abnormalities include the ones involving mixed lineage leukemia (MLL) gene rearrangements, RUNX1/RUNX1T1 (RUNX1) fusion, and chromosome 16 (inv(16)) inversion. MLL has the worst prognosis (5-year OS: 47%) while RUNX1 and inv(16) have much better prognoses (5-year OS: 91% and 87% respectively) ( von Neuhoff et al., 2010). Using single-cell profiling, our group had developed gene signatures to discriminate between these three subtypes, with the identified genes in the MLL signature correlating with a worse prognosis ( Krishnan et al., 2022). Here, to further improve the robustness of the blast-associated signatures, we employed a machine learning model and included more healthy control samples. Revealing differences between these subtypes' immune microenvironments (IME) is critical for identifying the cellular/molecular drivers of the poorer prognosis associated with the MLL subtype. To identify subtype-specific differences in genes/pathways, we performed an in-depth analysis of the blasts and IME.
Methods: We analyzed single-cell transcriptome data from pediatric AML bone marrow (BM) samples collected at diagnosis (MLL, n=4; RUNX1, n=4; inv(16), n=3) and healthy BM samples (n=9) ( Bailur et al., 2020; Caron et al., 2020). Quality control and filtering yielded >63,000 cells. The 7-gene signature from Thomas et al. (2020) was used to distinguish leukemic blasts from non-blast cells. Differential expression (DE) analysis was conducted using Wilcoxon's test between the three subtypes' blast cells and healthy myeloid cells (>50% subtype-blasts expression, <20% other samples' expression). The most differentially expressed genes (Log2FC>0.6, p<1e-5) were used to derive the subtype-specific gene signatures, and they were then further refined by considering only the genes that were also differentially expressed (p<1e-5) in the TARGET-AML bulk RNA-seq dataset ( McNeer et al., 2019). These signatures were validated by training a machine-learning-based LightGBM classifier ( Ke et al., 2017) on the gene expression levels in additional TARGET bulk RNA-seq data to differentiate between the three subtypes by themselves as well as other AML and healthy samples. Cell communication and gene regulatory analysis were performed using the CellChat ( Jin et al., 2021) and pySCENIC ( Aibar et al., 2017) tools respectively.
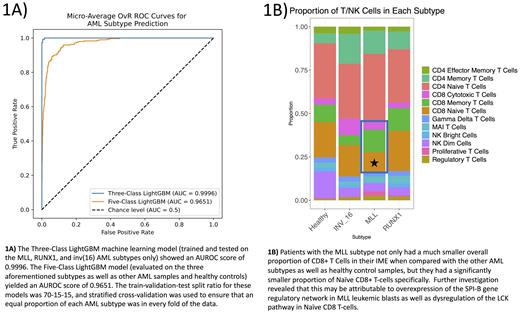
Results: Our analysis confirmed overexpression of the previously reported SOCS2, KCNE5 and TRPM4 genes in MLL leukemic blasts and identified new markers including LAMP5, LTC4S and HOXA9. HOXA9 is a transcription factor that has been shown to result in a poorer prognosis for patients suffering from AML ( Collins et al., 2015). CAV1 was found to be overexpressed in RUNX1-subtype blasts in addition to the POU4F1, NPW and TRH genes found previously. The inv(16) blast signature comprised of the AUTS2, PAM, ST18, SPARC and TSC22D1 genes. The LightGBM classifier trained on these signatures exhibited an overall AUROC test score of 0.9996 when discriminating between just the three subtypes and 0.9651 when other AML subtypes and healthy controls were considered as well (Fig. 1A).
In the T-cell compartment of the IME, MLL patients not only had a significantly (p<1e-5) lower proportion of CD8+ Naïve T-cells than any of the other subtypes but also had a significantly (p=0.026) smaller overall proportion of CD8+ T-cells (Fig. 1B). Gene regulatory analysis revealed that MLL leukemic blasts had significantly (p<2e-16) enriched activity of the SPI-B transcription factor (TF), known to suppress CD8+ T-cell proliferation in various cancers ( Huang et al., 2021). Additionally, cell communication analysis revealed significantly (p<0.01) less communication involving the LCK pathway within CD8+ Naïve T-cells, a pathway known to play a key role in proliferation ( Seddon et al., 2000)
Conclusions: This study evaluated the differences between the MLL, RUNX1, and inv(16) AML subtypes at the single-cell level, discovered gene signatures that effectively discriminate between the three subtypes as well as other AML and healthy samples, and revealed that the poorer prognosis of the MLL subtype may be attributable to dysregulation of CD8+ T-cell proliferation caused by abnormalities in the SPI-B TF and the LCK signaling pathway.
Disclosures
Bhasin:Anxomics LLC: Current Employment, Current equity holder in private company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal