INTRODUCTION:
The use of pediatric-inspired regimens for the treatment of acute lymphoblastic leukemia (ALL) has now become the standard of care in adolescents and adults (AYA). One such regimen, CALGB 10403, had higher rates of event free survival (EFS) and overall survival (OS) compared with historical controls using adult ALL regimens. It was shown to be feasible and efficacious in fit patients up to the age of 40 (Wendy Stock et al., Blood 2019). In 2012, based on emerging data on the improved efficacy of pediatric-inspired regimens in AYA patients, CALGB 10403 was adopted as the standard-of-care regimen in this population at Karmanos Cancer Institute (KCI), an adult cancer treatment center. Here we report our single center, real world experience of using this regimen in a Philadelphia chromosome negative (Ph-) AYA population, approximately 10 years after its adoption.
METHODS:
We conducted a retrospective chart review of consecutive AYA (ages 18-39) patients with Ph- ALL treated with CALGB 10403 regimen between January 2012 and June 2023 at KCI. Patients who received alternative regimens due to distance from KCI were excluded. Baseline demographic, clinical and molecular characteristics were noted. High-risk cytogenetics were defined as KMT2A rearrangements, hypodiploidy with a chromosome number ≤43, and complex karyotype. Testing for Ph-like ALL was not available in most patients. Beginning in 2019, clonoSEQ assay was used to test for minimal residual disease (MRD), with MRD negativity defined at a threshold of less than 10 -4. Incidence of adverse effects and treatment related mortality were reported to describe safety. Complete response (CR) rate, OS and PFS were used as efficacy outcomes. Kaplan-Meier curves were used to estimate OS and PFS.
RESULTS:
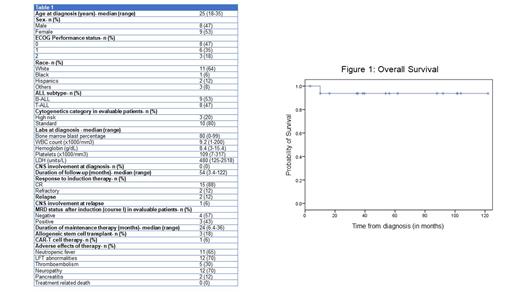
A total of 17 Ph- AYA patients were treated at KCI with a median age at diagnosis of 25 years (range 18-34). Males and females had a near equal representation (47% and 53%). The patients were predominantly White (64%). The majority of patients had a good ECOG performance status of 0 or 1 (88%). The diagnosis was B-ALL in 9 (53%) and T-ALL in 8 (47%) patients. High risk cytogenetics were present in 3 (80%) of the evaluable patients. None of the patients had CNS involvement at diagnosis. Baseline laboratory parameters expressed as medians were bone marrow blast percentage 80%, WBC count 9.2 x1000/mm 3, hemoglobin 8.4 g/dL, platelets 109 x1000/mm 3, and LDH 480.
The median duration of follow-up was 54 months. 15 (88%) patients had CR with induction, and MRD was negative in 4 (57%) of the 7 evaluable patients. 2 (12%) patients had primary refractory disease. One of the patients with refractory disease underwent further therapy with blinatumomab, inotuzumab, CAR-T and allogeneic stem cell transplantation and is alive at 16 months from diagnosis. Another underwent salvage with hyper-CVAD and allogeneic stem cell transplant, and is currently alive at 88 months from diagnosis. Two additional patients were MRD positive after induction (course I) but subsequently attained MRD negativity. One of these eventually relapsed 7.5 months from diagnosis with a mixed phenotypic (T/myeloid) leukemia and died at 10 months, while the other is currently under treatment. An additional patient with CR1 and unknown MRD status had CNS relapse at 35 months, underwent successful hyper-CVAD, reintroduction of intrathecal chemotherapy and allo-HSCT salvage and is alive at 122 months. The 5-year PFS and OS were 74.5% and 94% respectively and the medians were not reached for both.
Noteworthy adverse effects include neutropenic fever (65%), liver function tests (LFT) abnormalities (70%), and neuropathy from vincristine (70%). Peg-asparaginase administration was associated with pancreatitis in 12%, thromboembolism in 30% and hypersensitivity reactions in 24% requiring switching to alternative forms of asparaginase products (erwinia and Rylaze). These toxicities were manageable and there were no treatment-related deaths in our cohort.
CONCLUSIONS:
The pediatric-inspired regimen CALGB 10403 was safe and efficacious in our AYA Ph- ALL population with no deaths due to treatment and a 5-year overall survival of 94%. Toxicities, especially from peg-asparaginase, should be closely monitored. While most patients will attain durable remission/cure, the minority with refractory or relapsed disease can still be successfully salvaged to achieve long term survival.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal