Methotrexate (MTX) is an essential part of most chemotherapy regimens for acute lymphoblastic leukemia (ALL). It is administered intrathecally (IT), intravenously, and orally during ALL therapy. We performed a retrospective chart review to assess the central nervous system (CNS) manifestations induced by MTX in all children who received ALL therapy at our institution between July 2013 and June 2023. A list of patients with the ICD-9/10 codes for ALL or ALLy was obtained from the Electronic Medical Records for the study time period. Data collected included demographics, cytogenetics, laboratory test results, MTX pharmacological data if available, and details of the treatment of CNS manifestations attributable to MTX. Lastly, we had a single neuroradiologist re-review all the brain Magnetic Resonance Imaging (MRI) obtained in patients with MTX induced CNS manifestations.
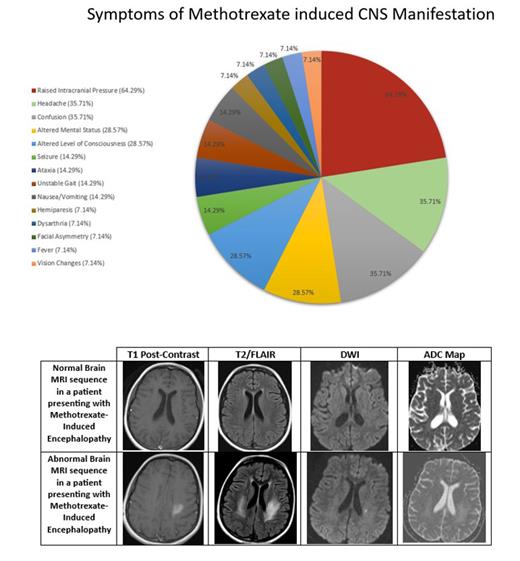
Sixty-five children were treated for ALL during the study decade. The average age at diagnosis was 4.7 ± 4 years. Our cohort consisted of 38 males and 27 females. Twelve of 65 patients (19%) developed MTX-induced CNS manifestations. The average age at the onset of symptoms was 5.3 ± 2 years. Ten patients had received a dose of IT MTX within 2 weeks of experiencing symptoms. Symptoms included raised intracranial pressure (ICP) (64%), headache (36%), confusion (36%), altered mental status (29%), and altered level of consciousness (29%).
Treatment of MTX-induced CNS manifestations consisted of therapeutic drainage of cerebrospinal fluid (CSF) with their scheduled IT chemotherapy administration (65%), acetazolamide (57%), theophylline + leucovorin + dextromethorphan (64%), and addition of hydrocortisone with IT medications (36%). In 3 (25%) patients the subsequent doses of MTX were delayed. The symptoms of CNS manifestations resolved in 100% of cases and MTX was able to be readministered in all the patients. Only one patient had recurrence of CNS manifestations twice after rechallenging with MTX; however, with specific symptomatic treatment, was able to tolerate the remainder of the IT therapy inclusive of MTX.
Brain MRIs were obtained in all patients. Initial imaging studies yielded normal results in 6 (50%) patients. Abnormal findings included subcortical white matter changes, vividly observed on T2/FLAIR MRI. These changes were represented by regions of acute hyperintensity seen in diffuse bilateral cerebral hemispheres (33%), parietal regions (33%), frontoparietal regions (17%), occipital regions (17%) and the left parietooccipital region (17%). A follow up MRI was performed 3 to 8 months after the initial MRI and was repeated up to 6 times over a course of 6 years in a patient. The earliest MRI improvement was evident after 3 months of initial imaging.
About 20% of patients developed MTX induced CNS manifestations in our cohort. IT MTX was the most common culprit. Raised ICP, on opening pressure measured during IT procedures happened to be the most common finding. This has not widely been reported previously under MTX induced CNS complications in published data. One of the suspected etiologies is the inflammation of arachnoid villi impairing CSF drainage. These patients responded well to acetazolamide and therapeutic drainage of CSF. Finally subcortical while matter changes on T2/FLAIR MRI was a common CNS manifestation.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal