Background: Asparaginases are enzymes that deplete circulating asparagine and glutamine from plasma and can therefore be used therapeutically for cancers that are sensitive to amino acid depletion. Commercially available asparaginases are isolated fromeither E. coli or Erwinia chrysanthemi (also called crisantaspase), the latter having a higher glutaminase activity. Our previous studies have shown that pegcrisantaspase (PegC), a long-acting pegylated crisantaspase, synergizes with the BCL2 inhibitor, Venetoclax (Ven), to kill several AML cell lines and patient-derived primary AML cells with complex karyotype in vitro and in vivo in a xenograft mouse model. Whether long-acting E. coli-derived asparaginases will have similar anti-AML activity as a single agent or in combination with BCL-2 inhibition, and the potential of asparaginases to synergize with inhibitors of MCL1, a key resistance factor of BCL2 inhibition, has yet to be determined.
Methods: Using a panel of 4 human AML cell lines (MOLM14, MV411, MonoMac6, HL60) as well as primary AML patient samples, weestablished the anti-leukemic activity of the BCL2 inhibitor S55746 and the MCL1 inhibitor S63845 alone and in combination with the long-acting E. coli asparaginase, calaspargase pegol-mknl (CalPegA). To determine the ability of S55746 or S63845 with or without CalPegA to inhibit mRNA translation, an established mechanism of Ven-PegC anti-leukemic activity, we measured the dissociation of the translation initiation factor eIF4E from 4EBP1, a negative regulator of cap-dependent mRNA translation, which then allows the formation of translation initiation complexes. The in vivo anti-leukemic efficacy of S55746 and S63845 alone and in combination with CalPegA was tested using an orthotopic AML xenograft mouse model with luciferase-expressing MV411 cells.
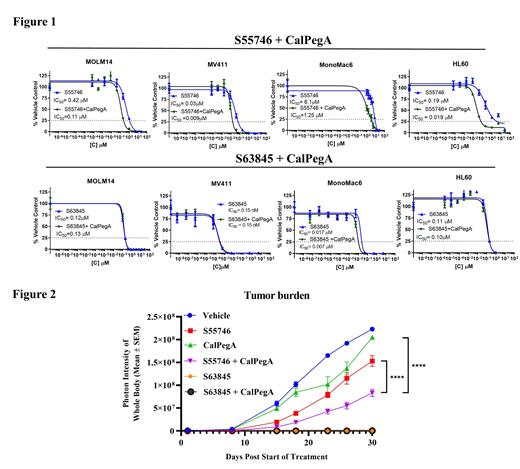
Results: We found that CalPegA treatment resulted in a dose-dependent inhibition of AML cell proliferation, with IC 50 values ranging from 0.78 to 2.8 IU/mL. Single agent S55746 and S63845 treatment inhibited AML cell proliferation with IC 50 values ranging from 0.01 to 6.7 µM and 0.0001 to 0.08 µM, respectively. In 3 of the human AML cell lines (MOLM-14, MV4-11, and MonoMac6), the addition of a small dose (IC 20) of CalPegA potentiated the anti-leukemic effect of S55746, reducing the IC 50 by approximately 1- 4-fold, and by 10-fold in HL60 cells (Figure 1). This potentiation was also observed in 3 out of 4 primary AML patient samples tested, with the IC 50 of S55746 reduced by 4.5, 18, and 156-fold by the addition of CalPegA. The activity of S63845 was not substantially impacted by the addition of CalPegA in either AML cell lines (Figure 1) or primary samples. Immunoprecipitation of 4EBP1 revealed that treatment with a combination of either S63845 or S55746 with CalPegA increased the binding of eIF4E to 4EBP1 compared to single agent treatments, suggesting an inhibition of translational complex formation. In a MV411 tumor-bearing model, treatment withS55746-CalPegA combination reduced tumor burden over time significantly more than either single agent. S63845 treatment was highly effective at inhibiting leukemia growth over time and was not improved by the addition of CalPegA, as tumor burden in the S63845 and S63845 + CalPegA-treated groups was not significantly different over the course of the study (Figure 2).
Conclusions: We report that the E. coli asparaginase, CalPegA, enhances the anti-leukemic effect of the BCL2 inhibitor, S55746, but does not impact the activity of the MCL1 inhibitor, S63845, in AML cell lines, patient derived primary AML samples, and in an AML xenograft mouse model. Ongoing studies are further investigating the anti-leukemic mechanism of S55476/S63845 + CalPegA and examining the efficacy and pharmacodynamic/pharmacokinetic effects of these agents in a patient derived xenograft model of AML.
OffLabel Disclosure:
Emadi:Secura Bio: Consultancy; NewLink Genetics: Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Servier: Research Funding; Kite Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Research Funding; KinaRx, Inc: Membership on an entity's Board of Directors or advisory committees, Other: Co-founder.
The presentation will discuss the use of calaspargase-pegol, which is indicated as a component of a multi-agent chemotherapeutic regimen for the treatment of acute lymphoblastic leukemia (ALL) in pediatric and young adult patients, in AML.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal