Introduction
Cancer-associated venous thromboembolism (VTE) is a major source of oncologic cost, morbidity and mortality. Identifying an optimal population for prophylactic anticoagulation is challenging and adds to clinician overhead. Circulating tumor (ct)DNA sequencing assays (“liquid biopsies”) are increasingly deployed in clinic, with multiple US FDA approvals for matching to molecularly targeted therapy. Preliminary data suggest that cell-free (cf)DNA, which is also measured as part of liquid biopsies and may consist of tumor or wild-type DNA, is thrombogenic due to its association with neutrophil extracellular traps. However, clinical validation of cfDNA for VTE prediction is lacking. ctDNA detection is associated with aggressive tumor biology and worse survival; however, whether it is associated with VTE is unknown. To address these gaps, we studied the clinical utility of ctDNA and cfDNA from liquid biopsies for predicting VTE.
Methods
We conducted an observational study in two cohorts: 1) Patients with any cancer type at Memorial Sloan Kettering Cancer Center (MSK, New York USA), an academic medical center, with plasma ctDNA sequencing using MSK-ACCESS, a NY State-approved, 129-gene assay (N=4,141). 2) A nonoverlapping cohort of patients at MSK or GenesisCare (Sydney, Australia), a community oncology setting, with stage IV or recurrent non-small cell lung cancer and ctDNA sequencing using a separate commercial assay (Agilent ctDx Lung, N=463). We studied the independent contribution of ctDNA, cfDNA and related variables for predicting VTE using Cox proportional hazards. We studied the potential utility of DNA liquid biopsies for predicting VTE within six months using machine learning models, comparing their dynamic area under the receiver operating curve, precision, and recall to those of the Khorana score, the most widely implemented risk metric. We tested the impact of ongoing anticoagulation for non-VTE indications on future VTE in patients with and without detectable ctDNA using nonrandomized, real-world evidence.
Results
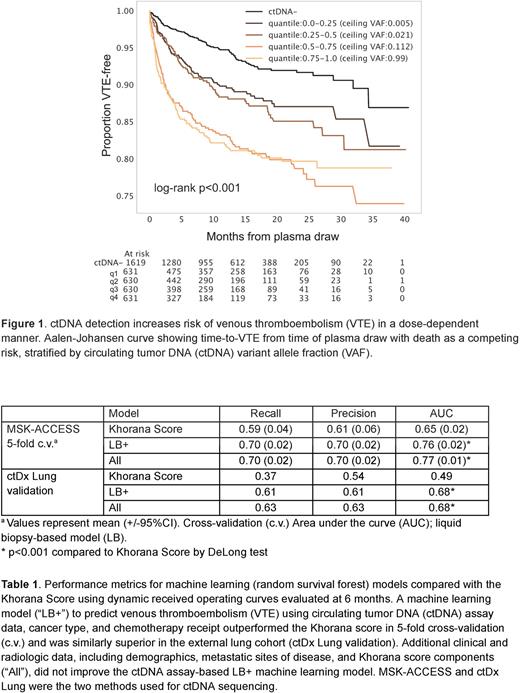
ctDNA detection was associated with an increased risk of VTE in a dose-dependent manner (Figure 1). This association was observed across assays and treatment sites and regardless of genomic subtype. ctDNA was associated with VTE risk in non-small cell lung, breast, pancreatic, prostate, and less represented cancers as well as melanoma but not in bladder, hepatobiliary, or colorectal cancer. By contrast, cfDNA concentration was associated with VTE risk in all cancer types. In multivariate analysis, ctDNA detection, cfDNA concentration, Khorana score, chemotherapy treatment within 30 days, and number of organ sites of metastasis were all independently associated with VTE. In a cohort of patients with treatment naive lung adenocarcinoma with segmented positron emission tomography scans, ctDNA was an independent predictor of VTE after controlling for metabolic tumor volume. A random survival forest machine learning model to predict VTE within six months of plasma draw using liquid biopsy data (i.e. cfDNA and ctDNA levels as well as genomic content), cancer type, and chemotherapy receipt (“LB+”) outperformed the Khorana score in 5-fold cross-validation and was similarly superior in the external lung cohort (Table 1). The superiority of the model was observed in patients treated or not treated with chemotherapy. Additional clinical and radiologic data, including demographics, metastatic sites of disease, and Khorana score components (“All”), did not improve the ctDNA assay-based LB+ model. Patients with anticoagulation had lower VTE rates if ctDNA was detectable (adjusted HR 0.54, 95%CI 0.33-0.87) but not if ctDNA was undetectable.
Conclusion
In patients with cancer, ctDNA and cfDNA are independent biomarkers for VTE. Risk models incorporating liquid biopsy data are feasible in academic or community settings and, if prospectively validated, would provide a means of VTE risk stratification at scale.
Disclosures
Fong:Various biotech exchange traded funds: Patents & Royalties: US20150181840A1. Guo:Agilent: Current Employment. Hernandez:Agilent: Current Employment. Garg:Agilent: Current Employment. Arcila:Janssen Global Services, Bristol-Myers Squibb, AstraZeneca, Roche, Biocartis: Consultancy; Biocartis, Invivoscribe, physician educational resources , Peerview institute for medical education, clinical care options, RMEI medical education: Speakers Bureau. Pavlakis:Boehringer Ingelheim, MSD, Merck, Bristol-Myers Squib, Astra Zeneca, Takeda, Pfizer, Roche, Novartis, Ipsen, and Bayer: Honoraria; Bayer, Pfizer, and Roche: Research Funding. Shah:Canesia Health Inc: Current equity holder in private company. Razavi:Grail, Illumina, Novartis, Epic Sciences, ArcherDx: Research Funding; Novartis, Foundation Medicine, AstraZeneca, Epic Sciences, Inivata, Natera, and Tempus: Consultancy. Reis-Filho:Roche, Genetech, Roche Tissue Diagnostics, Ventana, Novartis, InVicro, GRAIL, Goldman Sachs, Paige.AI and Volition RX: Membership on an entity's Board of Directors or advisory committees; Goldman Sachs, Paige.AI and REPARE Therapeutics: Consultancy. Ladanyi:Merck, Astra-Zeneca, Bristol Myers Squibb, Blueprint Medicines, Janssen Pharmaceuticals, Takeda Pharmaceuticals, Lilly Oncology, LOXO Oncology, Bayer, ADC Therapeutics, Riken Genesis, and Paige AI: Honoraria; LOXO Oncology, Merus, and Helsinn Therapeutics: Research Funding. Zwicker:Incyte Corporation, Quercegen: Research Funding; CSL Behring: Consultancy; Pfizer/BMS, Portola, Daiichi: Honoraria; Sanofi, CSL, Parexel: Consultancy; calyx: Consultancy; Sanofi: Consultancy; Janssen: Consultancy. Berger:Grail: Research Funding; PetDx and Eli Lilly: Consultancy. Li:Amgen, Genentech, AstraZeneca, Daiichi Sankyo, Lilly, Illumina, GRAIL, Guardant Health, Hengrui Therapeutics, MORE Health and Bolt Biotherapeutics: Research Funding. Mantha:Janssen Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal