Background: Introduction of C5 inhibitors is a major advancement in the treatment of Paroxysmal Nocturnal Hemoglobinuria (PNH) patients. First Eculizumab and then Ravulizumab significantly improved anemia by controlling intravascular hemolysis (IVH), reduced thrombosis risk, improved quality of life and prolonged survival. However, a significant proportion of PNH patients remain anemic with transfusion dependence despite maximal C5 inhibition due to C3 mediated residual extravascular hemolysis (EVH). Recently two randomized, open label, controlled trials with proximal complement inhibitors were studied in this patient population comparing against C5 inhibitors as single agents with excellent results. Here we compare efficacy and safety data of proximal complement inhibitors pegcetacoplan (C3 inhibitor), Iptacopan (factor b inhibitor) based on published or presented data.
Methods: We compared the efficacy and safety data of randomized portion of PEGASUS and APPLY-PNH trials. PEGASUS trial is a phase 3, randomized, open label, active-comparator controlled trial with Pegcetacoplan (a pegylated pentadecapeptide that targets C3) compared efficacy and safety against eculizumab in PNH patients with Hb <10.5 despite of treatment with Eculizumab (Hillerman, et al NEJM 2021;384). Primary end point of the study was mean change in Hb level from baseline to week 16. APPLY-PNH is an open label randomized, multicenter phase 3 trial compared Iptacopan 200 mg BID (oral factor b inhibitor) monotherapy with standard of care C5 inhibition therapy. (Regis P. de Latour et al. Blood (2022) 140 (Suppl 2): LBA-2). Primary end points of the trial were Hematological response defined as an increase in Hb of ≥ 2 g/dL from baseline and Hb ≥ 12 g/dL in absence of transfusions. Secondary end points included number of clinical and hematological markers of hemolysis and safety.
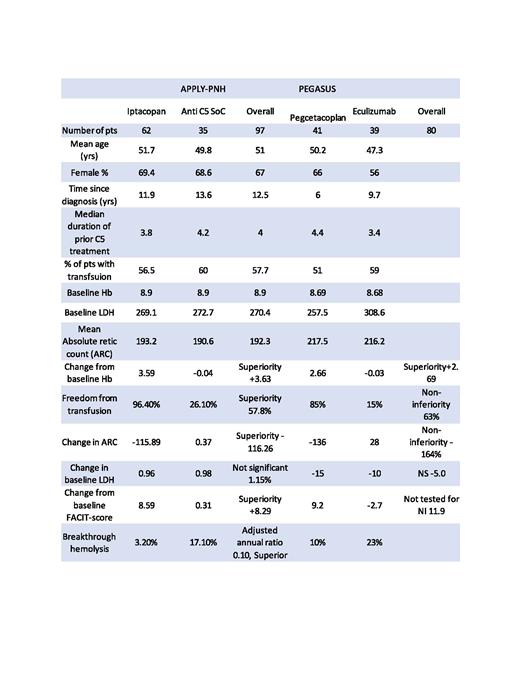
Results: Summarized in table 1. PEGASUS trial included 80 patients with 41 in pegcetacoplan arm and 39 in Eculizumab arm. APPLY-PNH included 97 patients with 62 in Iptacopan arm compared to 35 in standard of care anti C5 therapy. Majority of baseline characteristics were similar between two studies. Mean age in both studies was around 50 years and close to 2/3rd of patients in each study were females. Median duration of prior C5 therapy was between 3-4 years in both studies. More than half of patients in both studies required PRBC transfusions in preceding months prior to enrollment. Baseline mean Hb was between 8-9 g/dL, LDH between 250-300 U/L, mean absolute retic count (ARC) 190-200x109/L.
Both studies showed superiority in change from baseline Hb with +2.69 g/dL in PEGASUS trial with all available data regardless of transfusion events and +3.63 in APPLY-PNH trial. Both studies showed either noninferiority or superiority in freedom from transfusions and change in ARC. Interestingly, LDH change was not significant in both studies. Change from baseline fatigue FACIT-score was numerically better but not tested in PEGASUS trial and was superior in APPLY-PNH. No major vascular events or serious infections by encapsulated bacteria were reported in either study. Breakthrough hemolysis was reported in 4 out of 40 patients in PEGASUS trial that led to treatment discontinuation in 3 patients and 2 out of 62 patients in APPLY-PNH trial, however none of them required treatment discontinuation.
Conclusions:
PEGASUS and APPLY-PNH trials showed that in patients with persistent anemia despite C5 inhibitor therapy, proximal complement inhibition with either pegcetacoplan (C3 inhibitor) or Iptacopan (factor b inhibitor) as a single agent showed significant improvement in Hemoglobin with higher rate of transfusion independence with control of extra vascular hemolysis while maintaining intravascular hemolysis control with no serious infections. These studies represent another major advancement in the management of PNH patients. Long term follow up is needed regarding safety and to identify better ways to manage breakthrough hemolysis in patients on proximal complement inhibitors.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal