Introduction: Clonal Hematopoiesis of Indeterminate Potential (CHIP) is associated with cardiovascular disease, including acute myocardial infarction and ischemic stroke, due to immune system dysregulation leading to increased atherosclerotic plaque formation and inflammation. Approximately 25-40% of ischemic strokes have an unknown cause even after a comprehensive etiological study and are eventually categorized as cryptogenic strokes. Our hypothesis is that the presence of CHIP could represent a biomarker for atherosclerotic or cardioembolic ischemic stroke and help to clarify the origin of cryptogenic strokes. Therefore, the objective of this study was to analyze the prevalence of CHIP in patients with different subtypes of ischemic stroke and its prognostic impact on cardiovascular risk.
Methods: This prospective observational study included consecutive patients over 60 years of age with no prior history of neoplasia or cytotoxic treatment, who experienced embolic ischemic stroke between September 2021 and August 2022. Ischemic strokes were classified into cardioembolic (CE), atherothrombotic (AT) or cryptogenic (CRYP) etiologies after performing a systematic etiological study. During the stroke admission, peripheral blood samples were obtained in order to analyze the 13 genes most frequently associated with CHIP ( DNMT3A, TET2, ASXL1, JAK2, PPM1D, TP53, SF3B1, GNB1, SRSF2, CHEK2, CBL, GNAS, NRAS) through targeted next generation sequencing. Libraries were prepared using the KAPA HyperPlus capture chemistry (Roche) and were sequenced on a MiSeq platform (Illumina) following a 2x150bp paired-end read standard protocol, allowing the detection of variants with a variant allelic frequency (VAF) of ≥1%. The analysis was conducted using an in-house developed pipeline. Patients with one or more mutations in these genes with a VAF ≥2% were considered CHIP carriers. Cardiovascular risk factors including: hypertension, diabetes mellitus, dyslipidemia, smoking and obesity, were also analyzed.
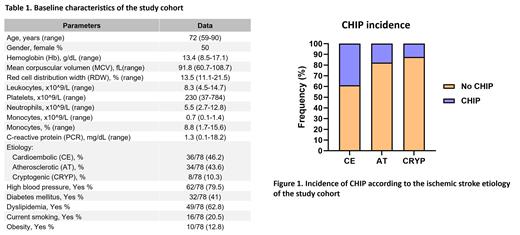
Results: Seventy-eight patients with stroke were included (50% female, median age 72 years (range: 59-90). Ischemic strokes were classified etiologically as: 36 CE (46.2%), 34 AT (43.6%) and 8 CRYP (10.3%) (Table 1). CHIP was detected in 21/78 (31.3%) patients, with the following gene mutation frequencies among CHIP carriers: DNMT3A (47.6%), TET2 (28.5%), SRSF2 (14.2%), PPM1D (9.5%), ASXL1 (4.8%), CBL (4.8%), CHEK2 (4.8%) and JAK2 (4.8%). The median VAF detected was 4.85% (range: 2.1%-43.7%), and 23.8% (5/21) of cases presented more than one mutation. The incidence of CHIP varied according to stroke etiology: CE etiology: 38.9% (14/36); AT etiology: 17.6% (6/34) and CRYP etiology: 12.5% (1/8) (p=0.046) (Figure 1). Moreover, only CE strokes showed mutations in TET2 (16.7% (6/36)) compared to AT (0/34) and CRYP (0/8) (p=0.015). No significant differences were observed in the blood counts (Hb, MCV, RDW, neutrophil count, monocytes, platelets or PCR) between stroke patients with CHIP and non-CHIP (Table 1). Finally, in the multivariate analysis, TET2 mutations were associated with CE stroke independently of age, coronary artery disease and classic cardiovascular risk factors (OR 8.1; Cl: 1.35-48.47; p=0.012).
Conclusions: Our findings identify that CHIP is common in patients with ischemic strokes, especially in those of cardioembolic etiology, with TET2 being the most frequently mutated gene. The presence of CHIP TET2 could represent a novel cardiovascular risk factor, contributing to a more precise etiological distinction between the different etiologies of ischemic stroke.
Funding source: this project is supported by health research fund of Carlos III health institute. FIS PI20/00881
Disclosures
Valcarcel:Pfizer: Consultancy, Other: Travel expense reimbursement, Speakers Bureau; Novartis: Consultancy, Other: Travel expense reimbursement, Speakers Bureau; Takeda: Consultancy; Kyte: Consultancy, Speakers Bureau; Astellas Pharma: Consultancy, Speakers Bureau; Sanofi: Consultancy, Speakers Bureau; GSK: Consultancy, Other: Travel expense reimbursement; Amgen: Consultancy, Other: travel expense reimbursement, Speakers Bureau; BMS/Celgene: Consultancy, Other: Travel expense reimbursement, Speakers Bureau; Jazz Pharmaceuticals: Consultancy, Other: Travel expense reimbursement, Speakers Bureau; Agios: Speakers Bureau; SOBI: Consultancy, Speakers Bureau; Gebro Pharma: Speakers Bureau; MSD: Consultancy, Speakers Bureau; Grifols: Speakers Bureau; Jansen: Speakers Bureau. Bosch:Roche: Honoraria; BeiGene: Consultancy; Lilly: Consultancy; Mundipharma: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria; Karyospharm: Other; Celgene: Consultancy, Honoraria; Roche: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal