Introduction
Emicizumab, a bispecific monoclonal antibody plays a crucial role in restoring adequate hemostasis by acting as a bridge between factors IX and X, effectively simulating the activity of factor VIII. Emicizumab is approved in several countries for bleeding episode prophylaxis in patients with HA with or without FVIII inhibitors regardless of severity.
This study aimed to report results from an assessment of tolerability, efficacy, and safety of Emicizumab prophylaxis in pediatric patients with HA at a single center in Saudi Arabia.
Methods This study was a retrospective study conducted between April 2019 and July 2023 at a Center for Blood Disorders for Pediatric Patients, Jeddah, Saudi Arabia.
Eligible study participants were individuals < 16 years of age, weighing over 3 kg, presenting severe or moderate congenital hemophilia A with or without FVIII inhibitors and those who were treated with Emicizumab for at least 3months at our center.
We decided to administer Emicizumab to patients with inhibitors, poor venous access, especially those of younger ages,and those with repeated breakthrough bleeds despite regular prophylaxis with FVIII concentrates. Parental drug preference was considered and discussed in all cases.
The initial 2 loading injections of Emicizumab (3 mg/kg once weekly for four weeks) were administered at the center, and the patients were observed for 1 h for signs of any acute adverse events. Following discharge, the caregivers of the patients were educated about proper medication administration techniques and instructed to contact our center in case of any adverse events.
Annual bleeding rate (ABR) were calculated at baseline pre and post-Emicizumab administration.Weight-based Emicizumab dosage adjustments were made at the end of each patient visit.
Results
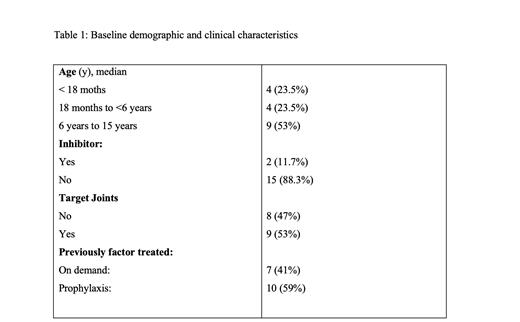
23 children with HA who started Emicizumab was initially included. A total of 6 patients were excluded as they didn't met the eligible criteria.10 patients with severe hemophilia (59%) and 7 patients with moderate hemophilia (41%). Patient were followed for median duration range of 217 weeks (15-232 weeks).
All study patients were males and median (range) age at enrollment was 8.0 (11 months to 16 years).All of them were treated previously with factor either on demand or as prophylaxis. Two patients presented with inhibitors, while the rest were the non-inhibitor type. Among these patients, 2 received a maintenance dose of 3 mg/kg of Emicizumab every 2 weeks, while 16 patients received a monthly dose of 6 mg/kg. Selection of regimen was based guardian preference and also approximate the dose to available drug concentration to avoid drug wastage.
Prior to enrollment, 9 patients had target joint involvement; among theses patients, 6 patients demonstrated improvement after Emicizumab initiation (66% reduction), while 3 patients still had persistent target joint involvement.
The initial baseline ABRs median (Range) was 48 (Range 5-120). Compared to the ABRs after initiation of Emicizumab which showed median of 2 (Range 0-18), the ABR mean value decreased from 38.5 at baseline to 4.6 after Emicizumabtreatment.
All bleeding events were traumatic and resolved with less than 48 h. one or two doses of Factor was/were required in four cases while bleeding was self-limited in others. 11 (64.7%) patients experienced zero bleeding.
We observed unusual side effect of asymptomatic persistent neutropenia was observed in 10 (58.8%) out of 17 patients. The absolute neutrophil count (ANC) ranged from 0.6 to 1.45 cells/µL in these cases.
There was no other adverse event was observed.
Two patients underwent a tooth extraction without post-surgery bleeding, and neither required factor VIII treatment. Additionally, one patient underwent facial laceration repair and received a prophylaxis dose of factor VIII, leading to a successful outcome without any bleeding during and after the procedure.
Conclusion
Our study demonstrates the effectiveness of Emicizumab prophylaxis for treating patients with moderate to severe HA.The participants reported high adherence to the treatment, improved satisfaction rates and the concept of subcutaneous injections was well accepted. Although neutropenia is considered a unusual side effect of this treatment, the treatment progressed smoothly and favorable safety profile was observed.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal