Emicizumab, a bispecific humanized monoclonal antibody, bridges activated factor IX (FIX) and FX to restore the function of missing activated FVIII in individuals with hemophilia A (PwHA). Prophylaxis with subcutaneous emicizumab promotes adequate hemostasis in PwHA. Emicizumab prophylaxis in children with hemophilia A has been approved in the United States for use in PwHA ages newborn and older, with or without FVIII inhibitors.
We report a 2-year-old boy with severe congenital hemophilia A who initially received prophylaxis with standard half-life factor VIII but later switched to subcutaneous emicizumab at 6 mg/kg every four weeks due to difficult IV access and parental preference. Shortly after starting emicizumab, the patient presented to the hospital with severe episodic epistaxis from the left nostril, requiring ICU admission and ENT consultation. Despite treatment, the condition did not improve, prompting the need for imaging studies. Subsequently, the patient developed ipsilateral eye proptosis and a mass protruding from the left nostril.
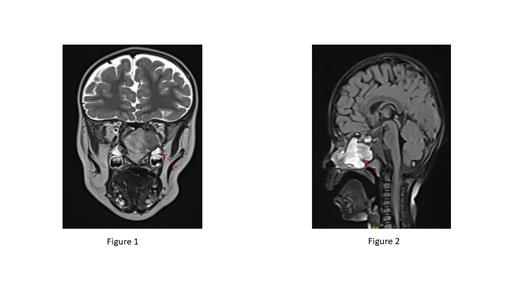
CT and MRI imaging of the orbit revealed an expansible soft tissue mass measuring 2 x 2.5 x 4 cm occupying the right sphenoid sinus, extending to the nasal cavity. The mass had caused erosion of the clivus and the anterior cranial fossa floor, extending to the middle cranial fossa (Figures 1 & 2).
He underwent endoscopic nasal debulking of the left nostril mass, and the pathological examination revealed a cystic mass with a thick capsule and intra-cystic blood clots, consistent with the characteristics of a hemophilic pseudotumor (HP). The fibrous stroma contained hemosiderin-laden macrophages with no evidence of malignancy.
Emicizumab was discontinued before the operation, and the patient received several doses of recombinant FVIII to maintain hemostasis. Despite the debulking procedure, the patient continued to experience episodic epistaxis and joint bleeding. An inhibitor assay was performed at that time and showed high levels, leading to a switch to on-demand FVIII treatment for hemophilia A with an inhibitor. Managing the bleeding episodes with bypassing agents proved challenging, and the family expressed dissatisfaction. A decision was made to resume emicizumab treatment, and the patient eventually stabilized, experiencing no bleeding for nearly six months. Additionally, he regularly follows up with ENT specialty, with no further intervention or progression of the tumor. Notably, he did not receive any bypassing agents concomitantly with emicizumab.
HP is characterized by encapsulated blood clots accumulating in extravascular tissues, especially the musculoskeletal system, in patients with bleeding tendencies, particularly hemophiliacs. It is a benign, expansible, soft tissue-like mass that exerts pressure on surrounding structures, mimicking a locally invasive malignancy. HP is a hematoma that grows and causes bone erosion through repeated bleeding episodes.
A literature review revealed that HP is a rare hemorrhagic complication that develops in 1-2% of patients with severe hemophilia (Magallón et al. Am J Hematol. 1994). Its occurrence in patients receiving emicizumab prophylaxis is extremely rare. The first reported case involved a Japanese child with hemophilia who presented with a pseudotumor occupying the left maxillary sinus after repeated episodes of epistaxis. The mass caused a downward deviation of the ipsilateral eye and bulged into the buccal cavity. The patient received monthly prophylaxis with emicizumab at 6 mg/kg (Kawahara et al. Int J Hematol. 2022), but unlike our patient, he received bypassing agents concomitantly.
Our reported case is unique because HP developed independently of bypassing agent therapies, as the patient did not receive any bypassing agents before the tumor's development. The likely explanation is related to repeated bleeding insults to the same anatomical location, leading to the cumulative development of hematoma and HPs. Another possibility could be attributed to the known adverse thrombotic effects of emicizumab treatment.
Physicians treating hemophilia should remain vigilant for abnormal clinical signs, such as new masses in patients receiving emicizumab, which could indicate HP.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal