Background:
Myelofibrosis (MF) is a myeloid neoplasm characterized by splenomegaly, constitutional symptoms, and cytopenias. Notably, worsening cytopenias in MF patients is directly correlated with poor survival ( Coltro, et al. Blood Cancer J. 2022;12(8):116). The JAK1/2 inhibitor ruxolitinib (RUX) is effective in decreasing splenomegaly and improving symptom control at starting doses of 30-40 mg total daily dose (TDD), though drug-induced cytopenias often lead to significant dose reductions. Doses of ≤20 mg TDD are associated with decreased efficacy for spleen response, and doses of ≤10 mg TDD demonstrate minimal efficacy for spleen or symptom reduction ( Verstovsek, et al. Onco Targets Ther. 2014;7:13-21). Treating with less clinically effective doses may not be optimal in light of newer, less myelosuppressive JAK2 inhibitors that can be administered without dose reduction, regardless of baseline cytopenias. This study describes RUX dosing patterns in real-world community practice.
Methods:
This retrospective, observational study included deidentified data of adult patients from the IntegraConnect PrecisionQ database (~80% community practice) with MF treated with first-line RUX and with ≥2 office visits (with a criterion of 2 MF diagnosis codes reported within 180 days) from January 2016 to July 2022. Patients were stratified by baseline platelet count (PLT). Data on RUX TDD at initiation and at first dose modification were collected, with a focus on less clinically effective dosing of ≤20 mg TDD and ≤10 mg TDD.
Results:
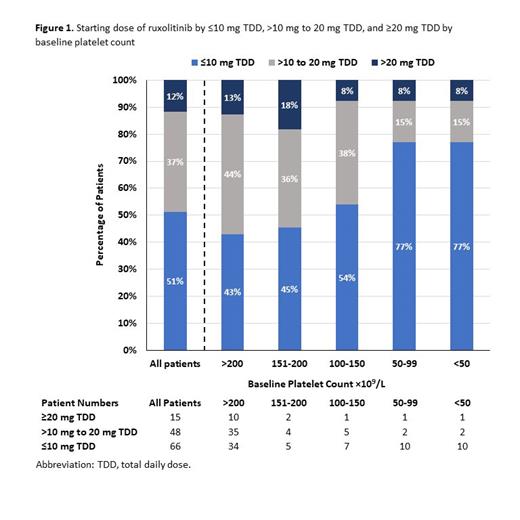
Of 129 patients on RUX with a median age of 75 (range: 42-89) years, 61% (n=79) had PLT >200x10 9/L, 19% (n=24) had PLT 100-200x10 9/L, and 20% (n=26) had PLT <100x10 9/L. Across all PLT strata, 88% (n=114) were initiated on RUX at a TDD of ≤20 mg, and 51% (n=66) were started at a TDD ≤10 mg. Nearly half of patients with higher baseline platelet counts (>100 x 10 9/L) were started on doses ≤10 mg TDD (Fig 1).
Among 79 patients with PLT >200x10 9/L, starting RUX TDD was ≤20 mg in 87% (n=69) and ≤10 mg in 43% (n=34). A total of 49 patients underwent subsequent dose modification (median time [min, max]: 47 days [10, 1336], with 19 dose increases vs 30 dose decreases. Of patients whose dose increased, only 4 escalated to a TDD >20 mg. Despite dose modification, RUX TDD remained ≤20 mg in 90% (44/49) and ≤10 mg in 43% (21/49) of patients. Similar to patients with baseline PLT >200x10 9/L, the majority of patients with PLT 100-200x10 9/L started on TDD ≤20 mg (87.5% [21/24]), with a high proportion on ≤10 mg (50% [12/24]).
Among the 103 patients with PLT >100x10 9/L for whom a recommended starting dose is provided in the RUX package insert, 88% (91/103) initiated RUX below the recommended dose. Of the 61 patients who underwent subsequent dose modification (median time: 47 days [10, 1336], only 10% (6/61) were escalated to a dose recognized as clinically effective.
Conclusions:
These real-world data suggest that a majority of patients starting RUX, including those with PLT >200x10 9/L, are initiated on ≤20 mg TDD, and the majority of patients are not titrated up to the doses tested in the pivotal studies that led to RUX approval. These data suggest that clinicians are hesitant to prescribe RUX at doses known to be clinically effective. Together with the timing of dose modification, this suggests that there may be concerns related to toxicity or treatment-related cytopenias. Recognizing that JAK2 inhibition remains an important part of managing MF for patients, clinicians now have alternative treatment options. These newer treatments inhibit additional pathways (IRAK1/ACVR1), are less myelosuppressive, and can maintain dose intensity regardless of baseline platelet count.
Study funded by CTI BioPharma Corp., a Sobi Company.
Disclosures
Rossetti:MPN Support Group: Speakers Bureau; Imago BioSciences, Inc., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA: Research Funding; Leukemia and Lymphoma Society: Membership on an entity's Board of Directors or advisory committees; Integra Connect: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Medical Writing, Speakers Bureau; CTI BioPharma Corp: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Medical Writing, Speakers Bureau; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Choksi:Integra Connect: Consultancy, Other: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events and support for attending meetings and/or travel . Suthar:CTI BioPharma Corp., a Sobi company: Current Employment, Other: Company provided vested and unvested equity awards to author as a company employee as part of overall compensation package, and all such equity grants were subject to accelerated vesting and pay out following Company's sale to new ownership. Davis:CTI BioPharma Corp., Sobi company: Current Employment, Other: Company provided vested and unvested equity awards to author as a company employee as part of overall compensation package, and all such equity grants were subject to accelerated vesting and pay out following Company's sale to new ownership; Eli Lilly and Company: Current equity holder in publicly-traded company, Ended employment in the past 24 months. Vasudevan:IntegraConnect, Precision Q: Current Employment. Dave:Integra Connect: Current Employment. Vaidya:Integra Connect: Current Employment. Wang:IntegraConnect, Precision Q: Current Employment. Katzen:Integra Connect: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal