Introduction: TKIs are the standard of care for CML-CP. Although effective TKI options are available in early lines of therapy of CML, patients may experience intolerance or resistance to TKI leading to non-optimal treatment (NOPT). This retrospective cohort study assessed the incremental healthcare resource utilization (HRU) and medical costs associated with NOPT in patients with CML treated in first-line (1L) or second-line (2L) TKI therapy in the US.
Methods: Adult patients with CML who received 1L TKI therapy with imatinib, dasatinib, nilotinib, or bosutinib (1L cohort), and those who received 2L TKI therapy with imatinib, dasatinib, nilotinib, bosutinib, or ponatinib (2L cohort), in 2012 or later, were selected from the OptumInsight Clinformatics database (01/2007-06/2022). Patients were required to have ≥2 years of continuous health plan coverage post-1L/2L initiation (1L/2L index date). Patients were classified in NOPT subgroup if they met one of the following criteria: 1) treatment discontinuation/switch within the first 6 months post-index, or 2) temporary treatment interruption and/or dose reduction within the first 6 months post-index followed by treatment discontinuation within the first 12 months post-index, or 3) low treatment adherence (proportion of days covered [PDC] ≤50%). Patients were classified in the reference (REF) subgroup if they met one of the following criteria: 1) high treatment adherence (PDC >90%) and no treatment discontinuation, 2) high treatment adherence (PDC >90%) and treatment discontinuation occurring more than 12 months post-index. All-cause HRU (inpatient [IP] admissions, outpatient [OP] visits, and emergency department [ED] visits) and associated medical costs (2022 USD) were measured during the 2 years post-index and reported per-patient-per-year (PPPY). Incremental HRU and costs of NOPT (vs. REF) were estimated using multivariable Poisson regressions and two-part models (adjusted for age, gender, race, region, health plan type, time from CML diagnosis to index date, and Darkow Disease Complexity Index), respectively. Adjusted incidence rate ratios (IRR) and mean cost differences (Δ) were reported along with p-value, estimated using bootstrap technique. All analyses were conducted separately in the 1L and 2L cohorts.
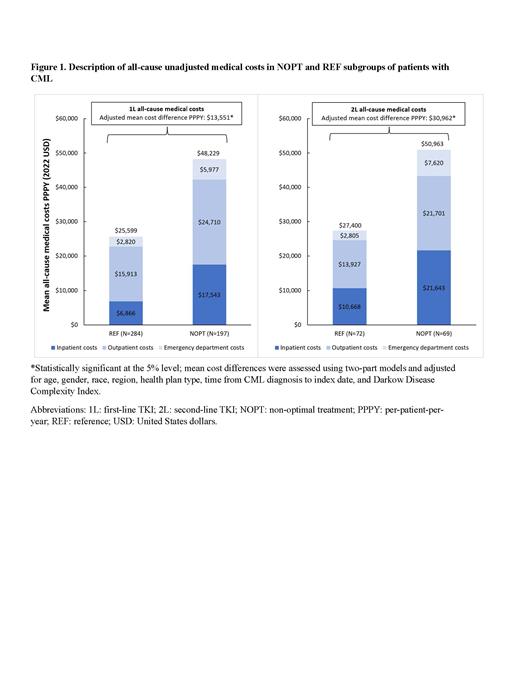
Results: Of 2,043 patients that initiated 1L TKI therapy in 2012 or later, 197 patients were included in NOPT subgroup (median age: 69 years; 52.3% female; 68.5% White) and 284 in REF subgroup (median age: 62 years; 40.1% female; 74.6% White). Patients in NOPT subgroup had 80% more IP admissions (adjusted IRR=1.8; p <0.001) with twice more IP days (adjusted IRR=2.0; p=0.024), 30% more OP visits (adjusted IRR=1.3; p=<0.001) in the first 2 years post-index, as compared to REF subgroup. Consistently, patients in NOPT subgroup had higher medical costs (adjusted Δ=$13,551 PPPY; p=0.012) mainly driven by higher IP costs (adjusted Δ =$5,989 PPPY; p=0.028) along with numerically higher OP costs (adjusted Δ=$5,483 PPPY; p=0.060) in the first 2 years post-index, as compared to REF subgroup ( Figure 1).
Of 586 patients that initiated 2L TKI therapy in 2012 or later, 69 patients were included in NOPT subgroup (median age: 67 years; 60.9% female; 76.8% White) and 72 in REF subgroup (median age: 68 years; 51.4% female; 69.4% White). Patients in NOPT subgroup had 3 times more IP admissions (adjusted IRR=3.1; p <0.001) with almost 7 times more IP days (adjusted IRR=6.7; p<0.001), 80% more ED visits (adjusted IRR=1.8; p=0.036), and numerically more OP visits (adjusted IRR=1.2; p=0.056) in the first 2 years post-index, as compared to REF subgroup. Consistently, patients in NOPT subgroup had higher medical costs (adjusted Δ=$30,962 PPPY; p=0.008) mainly driven by higher OP costs (adjusted Δ=$8,380 PPPY; p=0.016) and higher ED costs (adjusted Δ=$4,332 PPPY; p=0.012) along with numerically higher IP costs (adjusted Δ=$14,063 PPPY; p=0.100) in the first 2 years post-index, as compared to REF subgroup ( Figure 1).
Conclusions: In this study of patients with CML-CP non-optimal treatment resulting in early treatment discontinuation/switch or dose interruptions/reduction in the first 6 months from treatment initiation or low adherence with the currently approved TKIs in 1L and 2L therapy for CML was associated with significant incremental economic burden. This highlights the need for more tolerable therapeutic options early in the treatment course.
Disclosures
Kota:Kite: Honoraria; Novartis: Honoraria; Incyte: Research Funding; Pfizer: Honoraria. Wei:Novartis: Current Employment, Current equity holder in publicly-traded company. Yang:Novartis: Current Employment. Romdhani:Analysis Group Inc: Current Employment, Other: I am an employee of Analysis Group, Inc., a consulting company which received funding from Novartis.; Novartis Pharmaceuticals Corporation: Consultancy, Other: I am an employee of Analysis Group, Inc., a consulting company which received funding from Novartis.. Latremouille-Viau:Bristol Myers Squibb: Consultancy, Research Funding; Analysis Group, Inc.: Current Employment, Other: I am an employee of Analysis Group, Inc. a consulting company which received funding from Novartis.; Novartis Pharmaceuticals Corporation: Consultancy, Other: I am an employee of Analysis Group, Inc. a consulting company which received funding from Novartis.; Pfizer Inc.: Consultancy, Research Funding. Guérin:Novartis Pharmaceuticals Corporation: Consultancy, Other: I am an employee of Analysis Group, Inc. a consulting company which received funding from Novartis.; Analysis Group: Current Employment, Other: I am an employee of Analysis Group, Inc. a consulting company which received funding from Novartis.; AbbVie: Consultancy. Jadhav:Novartis: Current Employment, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal