Introduction
Venetoclax (ven) in combination with hypomethylating agents (HMA) is the standard of care for the treatment of acute myeloid leukemia (AML) in patients (pts) who are ineligible for intensive chemotherapy. The most serious complication of ven is tumor lysis syndrome (TLS). The phase 1b trial of ven and HMA in AML pts included strict prophylactic measures to prevent TLS. Reported rates of TLS in real world cohorts vary widely and prophylactic measures are not standardized.
Methods
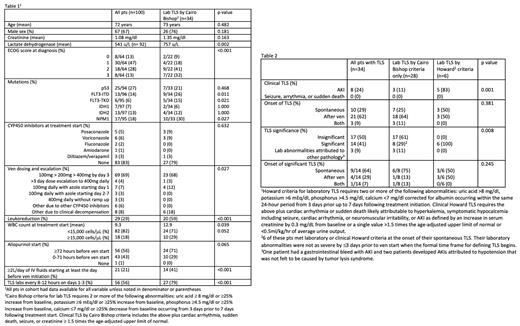
Here we describe TLS prophylaxis (ppx) and incidence in a cohort of 100 consecutive pts age ≥18 years at the University of Pennsylvania who received inpatient or outpatient ven and HMA for up front treatment of AML between 1/2017-8/2022. TLS ppx was at the discretion of the treating team. TLS was retrospectively defined by Cairo Bishop and Howard Criteria (table 1). Howard TLS (H-TLS) criteria differs from Cairo Bishop TLS (CB-TLS) in that it requires two lab abnormalities be present in the same 24-hour period, uses calcium corrected for albumin and does not include ≥25% changes from baseline lab values.
Results
92 pts had a white blood cell count (WBC) <25,000 cells/μL on the first day of treatment (table 1). 34 pts developed laboratory (lab) CB-TLS; 8 of these pts met criteria for clinical CB-TLS due to acute kidney injury (AKI). 13 pts developed CB-TLS prior to starting ven. While the original phase 1b trial mandated frequent lab monitoring as well as starting allopurinol ≥72 hours prior to ven and suggested a target of 2L of intravenous (IV) fluid per day starting the night prior to ven, TLS ppx was variable in our pts. 56 pts received allopurinol ≥72 hours prior to starting ven, 21 received ≥2L of IV fluid per day starting at least the day prior to ven, and 56 had TLS labs measured every 8-12 hours during the first 3 days following ven initiation.
CB-TLS was associated with a higher ECOG score and baseline lactate dehydrogenase as well as FLT3 and NPM1 mutations. The CB-TLS group had a higher mean WBC count but there was no difference in the proportion of pts with WBC counts ≥25,000 cells/μL. There was a trend towards increased CB-TLS in pts with WBC counts ≥15,000 cells/μL; this reached statistical significance when comparing the percent of pts with non-spontaneous CB-TLS with WBC count ≥15,000 cells/μL to the rest of the cohort (33% vs 13%, p=0.034).
To characterize TLS severity, we categorized the pts with CB-TLS into three groups: insignificant TLS, significant TLS, and those whose lab abnormalities were explained by a different pathology. We defined significant TLS as pts who required hospitalization (if started treatment outpatient) or ICU transfer, received rasburicase, developed AKI, or had treatment held due to TLS. 17 pts had insignificant TLS, 14 had significant TLS, and 3 were ultimately felt not to have TLS (table 2).
We also compared pts who developed CB-TLS with those who met criteria for H-TLS. Of the 34 pts who met lab CB-TLS criteria, only 6 also met Howard criteria. All 6 of these pts had significant TLS. Of the 8 pts with significant TLS who only met Cairo Bishop criteria, 6 developed spontaneous TLS >3 days prior to starting treatment with ven that would have met H-TLS criteria at onset. Their lab abnormalities were not as severe by ≤3 days prior to ven start when the formal time frame for defining TLS begins.
Discussion
Other studies have shown lab TLS rates of 0-40% in AML pts receiving ven with HMA, with the majority reporting rates <10%. Our data show a higher rate of lab CB-TLS than other published cohorts. However, our data also show that most pts do not develop significant TLS despite less aggressive ppx than was used in the original ven trial and suggest that less aggressive ppx is appropriate for most pts. There was a high rate of spontaneous TLS in our cohort. Debate exists over the most appropriate WBC threshold for ven initiation; our data suggest an association of WBC count <15,000 cells/μL with lower TLS risk. Of the pts who developed significant TLS, a majority had spontaneous TLS; only 5 pts had significant TLS occur after starting ven. We also found that the Howard criteria for TLS better identify pts who will have adverse outcomes from TLS and may be a more clinically meaningful set of criteria to use in future studies.
Disclosures
Carroll:Cartography Bioscences: Membership on an entity's Board of Directors or advisory committees; Janssen Pharmaceuticals: Consultancy. Frey:Kite Pharma: Consultancy; Sana Biotechnology: Consultancy. Gill:Kite Pharma: Consultancy; Carisma Therapeutics: Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties: patents, Research Funding; Interius Biotherapeutics: Current equity holder in private company, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Research Funding; Asher: Research Funding; Currus: Membership on an entity's Board of Directors or advisory committees; Inndura: Membership on an entity's Board of Directors or advisory committees; Mission Bio: Membership on an entity's Board of Directors or advisory committees; NKILT: Membership on an entity's Board of Directors or advisory committees; Vor Bio: Membership on an entity's Board of Directors or advisory committees, Research Funding. Lai:Genentech: Consultancy; Pfizer: Consultancy; Jazz: Consultancy, Research Funding, Speakers Bureau; Taiho: Consultancy; Novartis: Consultancy; Daiichi: Consultancy; BMS: Consultancy; Rigel: Consultancy; Astellas: Consultancy, Speakers Bureau; AbbVie: Consultancy. Luger:Onconova: Research Funding; Bristol-Myers Squibb: Honoraria; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Marker Therapeutics: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy; Astellas: Honoraria. Maillard:Garuda Therapeutics: Membership on an entity's Board of Directors or advisory committees; Genentech: Research Funding; Regeneron: Research Funding. Perl:Genentech: Honoraria; Daiichi-Sankyo: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer: Research Funding; Foghorn: Consultancy; Forma: Consultancy; BMS: Honoraria; Abbvie: Consultancy, Honoraria, Research Funding; BerGen Bio: Honoraria; Beat AML: Other: Participation on a Data Safety Monitoring Board or Advisory Board; Syndax: Research Funding; FujiFilm: Research Funding; Immunogen: Honoraria; Aptose: Honoraria; Rigel: Honoraria; Actinium: Honoraria. Porter:Sana Therapeutics: Consultancy, Current equity holder in publicly-traded company; Tmunity: Patents & Royalties; Wiley and Sons Publishing: Honoraria; Novartis: Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding; National Marrow Donor Program: Membership on an entity's Board of Directors or advisory committees; Mirror Biologics: Membership on an entity's Board of Directors or advisory committees; Kite/Gilead: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Genentech: Current equity holder in publicly-traded company; DeCart: Membership on an entity's Board of Directors or advisory committees; Capstan Bio: Honoraria; BMS: Membership on an entity's Board of Directors or advisory committees; Bluebird Bio: Membership on an entity's Board of Directors or advisory committees; Angiocrine Bio: Membership on an entity's Board of Directors or advisory committees. Pratz:Roche: Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees; Jazz Pharamceuticals: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Agios Pharmaceuticals: Research Funding; AbbVie: Consultancy, Research Funding. Stadtmauer:Amgen: Consultancy; Janssen: Consultancy; BMS: Consultancy; Abbvie: Consultancy, Research Funding; genmab: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal