Introduction
Monoclonal gammopathy (MG) is present in 4.5% of adults older than 40 years. Given an annual 1% rate of progression to multiple myeloma (MM), regular MG monitoring has the potential to improve MM outcomes through earlier diagnosis. To this end, a variety of surveillance systems have been implemented in the NHS, including hospital, primary care, and nurse-led surveillance. However, as the vast majority of new MG patients are low-risk and unlikely to progress, most services are inefficient and unsustainable to support population-based monitoring. There is a need for a robust high-throughput system that can monitor most patients in the community, whilst streamlining high-risk MG for review in secondary care. Here, we describe a risk-adapted MG monitoring program (OxCOM), in which patients with incidental MG in Thames Valley were prospectively stratified and monitored.
Methods
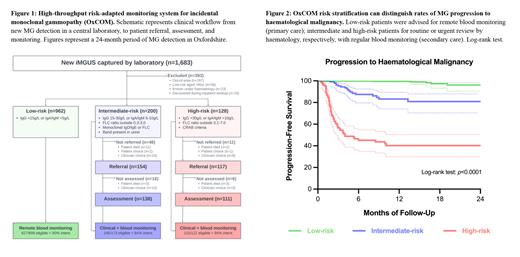
Patients with incidental MG were captured over a 24-month period (between 4 th March 2021 and 3 rd March 2023) in an Immunology Laboratory covering a population of 2.5 million people. An attending immunologist risk-stratified patients daily into high-risk [IgG >30g/L or IgA/IgM >10g/L; or free light chain (FLC) ratio outside 0.1-7.0; or CRAB criteria], intermediate-risk [IgG 15-30g/L or IgA/IgM 5-10g/L; or FLC ratio outside 0.3-3.0; or monoclonal IgD/E or FLC; or urinary band] or low-risk [IgG <15g/L or IgA/IgM <5g/L] MG. Patients were excluded from analysis if out-of-area, aged 90 years or older with low-risk MG, under regular haematology review or had malignancy diagnosed as an inpatient following MG detection. The laboratory advised the requesting clinician to refer low-risk patients for 4-12-monthly remote blood monitoring by primary care, supervised by an OxCOM administrator. Intermediate and high-risk patients were advised for routine or urgent clinical assessment under haematology, respectively, with blood monitoring in secondary care. Intent for monitoring was assessed by patients receiving subsequent surveillance within 4 months (IgA or IgM) or 12 months (IgG), with a 2-month grace period. Follow-up rates were compared to a reference cohort of new MG detected in 2019 (prior to setup of OxCOM, in which monitoring was managed in the community) that were risk stratified retrospectively.
Results
1,290 patients had MG detected over a 24-month period [962 low-risk (75%), 200 intermediate-risk (16%) and 128 high-risk (9%)] (Figure 1), of which 50% were female and median age was 75.4 years. Testing was requested equally between primary and secondary care (50% each); the most common indications were anemia (13%), bone pain (13%) and fracture (13%), all of which were over-represented in intermediate and high-risk MG [ p=0.0002]. Of 25% patients referred to secondary care [5% low-risk, 77% intermediate-risk and 91% high-risk], resources were concentrated towards higher-risk patients. Patients with high-risk MG were reviewed more quickly than those with intermediate-risk MG (median 14 vs. 54 days since test) [ p<0.0001], more likely to be assessed F2F (98% vs. 55%) [ p<0.0001] and receive investigations including CT/MRI imaging (86% vs. 44%) and bone marrow biopsy (59% vs. 17%) [ p<0.0001]. OxCOM risk stratification criteria distinguished risk of haematological malignancy diagnosis between low-risk (1%, n=10), intermediate-risk (14%, n=18) and high-risk (57%, n=57) MG at last known follow-up [ p<0.0001] (Figure 2). OxCOM enabled risk reclassification based on clinical judgement; of 253 patients initially assessed by secondary care, 65 (26%) had been transferred to remote primary care monitoring after a median of 3 clinical reviews and 7.3 months follow-up. There was a higher fidelity of subsequent monitoring at an appropriate interval under OxCOM (89%) than in the pre-OxCOM system (47%), including in those with low-risk (90% vs. 35%) and high-risk (94% vs. 76%) MG [ p<0.0001].
Conclusions
We demonstrate the feasibility of a risk-adapted high-throughput clinical monitoring service for incidental MG. Immunology Laboratory led central oversight by stratifying patients for either remote blood surveillance under primary care, or for clinical assessment in secondary care. OxCOM therefore establishes a model to enable tailored monitoring of a premalignant state, which distributes healthcare resource towards higher-risk patients, whilst enabling sustainable monitoring for those with lower-risk.
Disclosures
Gooding:Bristol Myers Squibb: Research Funding. Ramasamy:AbbVie, Adaptive Biotechnologies, Amgen, Celgene (BMS), GSK, Janssen, Karyopharm, Oncopeptides, Pfizer, Sanofi, Takeda, Recordati pharma, Menarini Stemline: Speakers Bureau; AbbVie, Adaptive Biotechnologies, Amgen, Celgene (BMS), GSK, Janssen, Karyopharm, Oncopeptides, Pfizer, Sanofi, Takeda Recordati pharma, Menarini Stemline: Honoraria; Amgen, Celgene (BMS), GSK, Janssen, Takeda: Research Funding; Pfizer, GSK: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal