Introduction:
NGS is increasingly being utilized to identify pathogenic somatic and targetable variants in patients with newly diagnosed AML and MDS. Patients with high-risk disease are typically recommended to undergo allo-HCT as consolidation, upon achieving morphological complete remission (CR). But, despite achieving hematologic CR, patients often have detectable somatic variants pre allo-HCT that predict for increased risk of disease relapse. Longitudinal monitoring of somatic variants via NGS at diagnosis, pre-allo-HCT and post allo-HCT can assist in prognostication and identify patients that remain at high risk of relapse despite allo-HCT.
Methods:
Patients with AML or MDS who underwent a first allo-HCT and completed NGS testing at day +100 between years 2016 to 2021 were identified from the transplant registry of Mayo Clinic in Florida. The primary aim of the study was to evaluate the prognostic impact of detectable variants via NGS at day +100 days post allo-HCT on the following outcomes: 1) overall survival (OS), 2) relapse, 3) progression-free survival (PFS), and 4) non-relapse mortality (NRM). Disease risk was assessed based on European Leukemia Network (ELN) 2022 risk classification for AML and Revised International Prognostic Scoring System (R-IPPS) for MDS. Baseline variables were compared between patients with and without mutation at day +100 days post allo-HCT using Wilcoxon rank sum test for continuous variables and Fisher's exact test for categorical variables. Kaplan-Meier (KM) method was used to estimate freedom from events at different time points and draw corresponding survival curves.
Results:
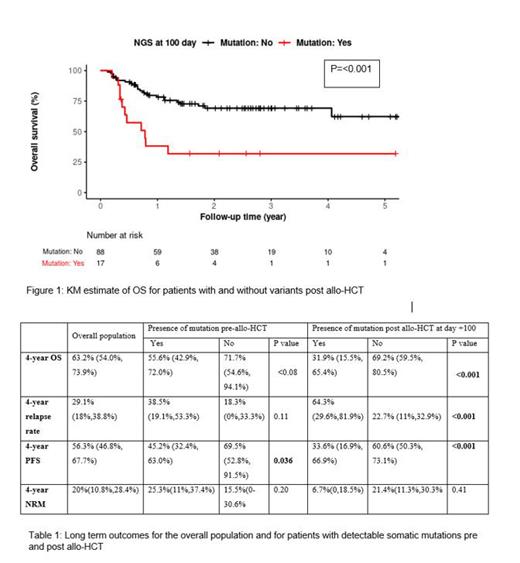
105 patients who underwent allo-HCT for AML and MDS and completed NGS testing at day +100 were included in the study population. Median age of the study population was 63 years (22-74) and 58% were of the male gender. Median HCT comorbidity score (HCT-CI) and KPS Karnofsky performance score (KPS) were 3 (0-8) and 90 (60-100), respectively. Matched unrelated donor (50%) and peripheral blood stem cells (98%) were the most common donor and graft source, respectively. Median number of lines of therapy pre allo-HCT was 1(0-6) and 52 (50%) patients had detectable variants after completion of chemotherapy and prior to allo-HCT. Seventeen (16%) had detectable variants via NGS on day +100. Eleven (65%) of these 17 had variants pre-allo-HCT and amongst the 88 patients with no variants post allo-HCT, 41 (47%) had detectable variants pre allo-HCT. There was no significant difference in baseline characteristics between patients with detectable variants at day+100 versus those who did not except for a higher KPS in patients with no variants at Day +100 of 90 (60-100) vs. 80 (60-100) (p=0.003). At a median follow up of 1.4 years (0.1-5.5), presence of a variant at day +100 was associated with inferior 4-year OS (32% vs. 69%, p<0.001) and higher relapse risk (64% vs. 23%, p<0.001). In univariate models, a lower KPS and presence of variants post allo-HCT were significantly associated with inferior OS, high relapse risk and worse PFS. Presence of variants pre allo-HCT was associated with an inferior PFS but did not impact OS or relapse risk. In multivariate models, KPS remained significant for OS, PFS and NRM; presence of variants at day +100 post allo-HCT approached significance for OS (p=0.06) and PFS (p=0.08) and was significant for relapse risk (p=0.002) (Table1) (Figure 1). Data regarding longitudinal evolution of variants and impact of individual variants on long term outcomes will be included at the time of presentation.
Conclusion:
These results indicate that presence of detectable somatic variants at day +100 is associated with poor long-term outcomes and predicts for high risk of disease relapse. Longitudinal monitoring of somatic variants via NGS should be consistently incorporated in the management of patients with high-risk AML and MDS undergoing allo-HCT. Patients with detectable somatic variants post allo-HCT should be considered for additional intervention to mitigate relapse risk.
Disclosures
Murthy:Novartis: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Senti Biosciences: Membership on an entity's Board of Directors or advisory committees; CRISPR Therapeutics: Membership on an entity's Board of Directors or advisory committees; Bavarian Nordic: Membership on an entity's Board of Directors or advisory committees. Foran:BeiGene: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Actinium: Research Funding; Kura: Research Funding; Sellas: Research Funding; Roivant: Research Funding; Novartis: Research Funding; Celgene: Research Funding; Astellas: Research Funding; NCI: Membership on an entity's Board of Directors or advisory committees; CTI: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal