Introduction: Iptacopan is a first-in-class, oral, selective complement factor B inhibitor that recently demonstrated efficacy and safety as monotherapy in both C5 inhibitor-treated (APPLY-PNH [NCT04558918], Peffault de Latour et al) and treatment-naïve (APPOINT-PNH [NCT04820530], Risitano et al) patients with paroxysmal nocturnal hemoglobinuria (PNH). The primary results of active-comparator controlled APPLY-PNH and single-arm APPOINT-PNH have been reported, showing clinically meaningful increases in hemoglobin levels while controlling intravascular hemolysis and preventing extravascular hemolysis. As patient-perceived benefits of therapies are increasingly important, we present here additional results of the patient-reported outcomes measures (PROMs) including responder analyses of changes from baseline in these two studies based on meaningful within-patient thresholds.
Methods: The PROMs included an assessment of fatigue (FACIT-Fatigue) as the basis of a secondary endpoint, a health-related quality of life questionnaire (EORTC QLQ-C30), and a Patient Global Impression of Severity (PGIS) of Fatigue. Both studies analyzed change from baseline in PROMs between Day 1 and Day 168. In APPLY-PNH, another focus was on comparing treatments on the average of model-derived estimates for visits between Day 126 and Day 168. While 5 points on the FACIT-Fatigue was prespecified in the protocol, anchor-based meaningful within-patient change analyses utilizing the PGIS as the anchor determined the range for the magnitude of meaningful change for the FACIT-Fatigue to be 7.5 to 9.5 points (derived from APPLY-PNH); 9 points within the upper segment of the range was chosen as the threshold value used in this analysis. Analyses of the EORTC QLQ C-30 focused on the Physical and Role Functioning subscales and symptoms subscales evaluating Fatigue and Dyspnea. Responder analysis thresholds were determined for all 4 subscales using a combination of anchors including PGIS to determine meaningful change thresholds for each subscale. The ranges and threshold choices were based on the assessment of empirical cumulative distribution function (eCDF) of the scores by scale levels of the anchor and used the visit median values as representative levels to determine the range supporting threshold choices.
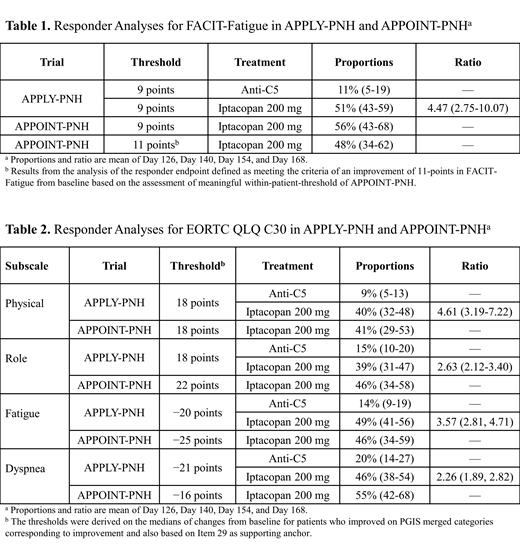
Results: Ninety-five patients were from APPLY-PNH, of whom 62 received iptacopan and 33 received anti-C5 treatment, and 40 patients were from APPOINT-PNH. In APPLY-PNH, responder analysis showed that more patients in the iptacopan group achieved the threshold of ≥9 points compared with the anti-C5 group (51% vs 11%) ( Table 1). The strength of this result was confirmed by a non-parametric Mann-Whitney test with P values ≤0.0039. In APPOINT-PNH, 56% (95% CI, 43-68) achieved the threshold of ≥9 points and approximately half reached a higher threshold of 11 points ( Table 1). Analyses of EORTC QLQ-C30 further corroborated the results for both trials. Responder analyses in APPLY-PNH of the four subscales using internally derived thresholds showed average proportions of responders in the iptacopan group 39% to 49% and in the anti-C5 group ranging from 9% to 20% ( Table 2). The strength of these results was confirmed by a non-parametric assessment of cumulative distribution functions and Mann-Whitney with P values ≤0.0088. In APPOINT-PNH, the estimated average proportions of responders for EORTC QLQ C30 ranged from 41% to 55% ( Table 2).
Conclusion: Both anti-C5-experienced and treatment-naïve patients receiving iptacopan exhibited clinically meaningful improvements in fatigue, health-related quality of life, and disease-related symptoms as measured by predetermined meaningful thresholds of response in FACIT-Fatigue and EORTC QLQ-C30. Together with previous presented primary efficacy data from APPLY-PNH and APPOINT-PNH, these results indicate that iptacopan not only results in clinically meaningful improvements in markers of disease control but provides meaningful improvements in aspects that are important to patients (ie, fatigue and other aspects of health-related quality of life, such as physical functioning and role functioning).
Disclosures
Risitano:F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Research Funding; Alexion, AstraZeneca Rare Disease: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria. de Castro:Omeros: Other: advisory board; Novartis: Consultancy, Honoraria, Other: Medical steering committee; advisory board; Alexion: Consultancy, Honoraria, Other: Advisory board, Research Funding, Speakers Bureau; Biocryst: Consultancy, Honoraria; Apellis: Consultancy, Honoraria, Other: Advisory board, Research Funding, Speakers Bureau; Regeneron: Other: Data safety monitoring board; Bristol Myers Squibb: Speakers Bureau. Kulasekararaj:F. Hoffmann-La Roche Ltd: Consultancy, Membership on an entity's Board of Directors or advisory committees; BioCryst: Consultancy; Samsung: Consultancy; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene/BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Akari Therapeutics: Consultancy; Achillion: Consultancy; Alexion, AstraZeneca Rare Disease: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Maciejewski:Alexion: Membership on an entity's Board of Directors or advisory committees; Regeneron: Consultancy, Honoraria; Novartis: Honoraria, Speakers Bureau; Omeros: Consultancy. Scheinberg:Viracta: Research Funding; Pfizer: Consultancy, Other: Speaker, Research Funding; F. Hoffmann-La Roche Ltd,: Consultancy, Other: Scientific presentations, Research Funding; Janssen: Consultancy, Other: Scientific presentations/speaker; BMS: Other: Speaker; BioCryst: Consultancy, Research Funding; AstraZeneca: Consultancy, Other: Scientific presentations/speaker, Research Funding; Amgen: Consultancy, Other: Scientific presentations/speaker; Alnylam: Research Funding; Alexion: Consultancy, Other: Scientific presentations/speaker; Novartis: Consultancy, Other: Scientific presentations, Research Funding, Speakers Bureau; AbbVie: Consultancy, Other: Speaker. Ueda:SOBI: Consultancy, Honoraria; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy; Chugai: Consultancy, Honoraria, Research Funding; Asahi Kase: Consultancy; Alexion: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Vallow:Novartis Pharmaceuticals Corporation: Current Employment, Current equity holder in publicly-traded company. Bermann:AstraZeneca: Current holder of stock options in a privately-held company; Novartis Pharma AG: Current Employment, Current holder of stock options in a privately-held company. Dahlke:Novartis Pharma AG: Current Employment. Kumar:Novartis: Current Employment, Current equity holder in publicly-traded company. Peffault de Latour:Jazz Pharmaceuticals: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal