Background: Myelodysplastic Syndromes/Neoplasms (MDS) are characterized by somatic driver mutations in hematopoietic stem/progenitor cells (HSPCs) leading to hypermethylation, disruption of normal hematopoiesis and progression to acute myeloid leukemia ( Cazzola M, N Engl J Med 2020). With exception of a minority of fit patients, who are candidates for treatment with the potentially curative allogenic hematopoietic stem cell transplantation (allo HSCT), most elderly patients remain incurable and receive palliative treatment with hypomethylating agents. Due to the high phenotypic overlap between normal and dysplastic HSPCs, eradication of diseased cells remains an unmet therapeutic need for patients not eligible for allo HSCT.
Aim: We applied a multiomics profiling strategy to identify novel oncospecific protein isoforms as potential drug targets in HSPCs of MDS and MDS/MPN patients. We hypotesized that isoforms might be more specific biomarkers and targets than consensus RNA and proteins.
Methods: We included 91 individuals with MDS and MDS/MPN at diagnosis or follow-up without disease modifying treatment (n=71), unclear cytopenia (n=20) and healthy controls (n=6). All samples were collected through our Swiss MDS Registry/Biobank Platform between 8/2017 and 11/2021. We classified all patients clinically by WHO 2016 and genetically using the Malcovati criteria ( Malcovati L et al., Blood 2017). Targeted sequencing of 65 established myeloid driver genes from bulk DNA was performed using the Illumina sequencing platform. RNA and proteins were extracted from selected CD34/CD117 HSPC cells. RNA profiling was performed using libraries generated with the SMART-Seq mRNA reagent and proteotypic profiling was done by tandem mass-spectrometry. RNA isoforms were identified using StringTie ( Pertea M et al., Nature Biotechnology 2015) and protein isoforms using a reference database constructed from the identified RNA isoforms. RMATS was used to detect differences in alternative splicing events. Differential expression of RNA isoforms was analyzed using Ballgown (Frazee A et al. Nat Biotechnol 2015 ) and conventional differential gene-expression was performed by DESeq2 ( Love MI et al., Genome Biology 2014).
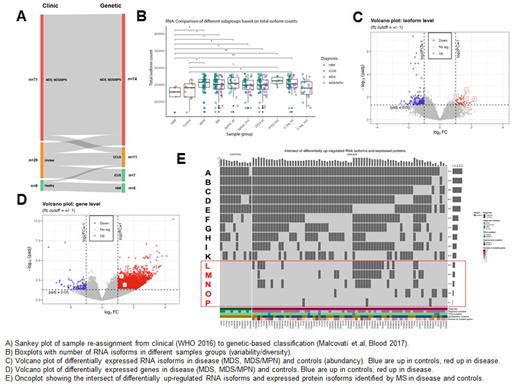
Results: Genetic classification identified relevant clonality in 2/3 of patients with clinically unclear cytopenia after morphological and cytogenetic assessment (6 MDS, 7 CCUS, 7 ICUS) ( A). The distribution of somatic driver mutations in our cohort was in line with published literature (data not shown). Compared to controls, patients with MDS and MDS/MPN showed a higher rate of intron retentions (data not shown), higher transcriptional diversity of RNA isoforms ( B), lower numbers of differentially over-expressed RNA isoforms (lower isoform abundancy) ( C) but higher numbers of differentially over-expressed genes (higher gene-expression abundancy)( D). This apparently controversial result reflects the methodological bias where RNA isoforms are merged on the corresponding gene-locus by conventional gene-expression analysis missing the full range of transcriptional (isoform) diversity. This RNA isoform diversity was not clearly associated with clinical (MDS vs MDS/MPN) or genetic subgroups (TP53, spliceosome or cell-signaling mutations). Due to low sample size, we could only observe a tendency of higher RNA isoform diversity in TP53 mutated and lower diversity in cell-signaling mutated subgroups ( C). The intersection of differentially up-regulated RNA isoforms and expressed protein isoforms revealed five novel oncospecific proteins (L-P in E) as potential candidates for specific drug targeting in HSPCs of MDS and MDS/MPN. These included an oncospecific membrane protein (L) as well as a cell-signaling enzyme (M) present in about 25%-30% of the diseased samples.
Conclusions: With our multiomics profiling strategy, we identified a higher RNA isoform diversity in HSPCs of MDS and MDS/MPN patients as well as five potential oncospecific protein isoform targets. These potential targets can be missed by differential gene-expression ( D) and are better discernible by the biologically more relevant differential RNA-isoform analysis ( C). L and M have well-established functions in stem-cell biology, leukemic transformation and drug resistance making them promising candidates for further functional validation as disease-specific drug targets.
Disclosures
Bonadies:Celgene/BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Astellas: Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sandoz: Membership on an entity's Board of Directors or advisory committees, Research Funding; Servier: Research Funding; Keros: Consultancy; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding. Rao:mAbTree Biologics: Consultancy; Protagonist Therapeutics: Consultancy, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal