Introduction
Peripheral T-cell lymphomas (PTCL) remain an unmet medical need and novel therapeutic options inducing longstanding responses are needed. Immunotherapies with humanized monoclonal antibodies targeting cell surface receptors have dramatically improved the prognosis of many cancers. However, so far, only two antibodies targeting CCR4 and CD30 have been approved for the treatment of subsets of PTCL patients. Currently, several bi- and tri-specific antibodies are under pre-clinical or clinical evaluation to treat lymphomas. TSAR442257, a trispecific antibody (TriAb) which targets and binds CD38, CD3 and CD28 to engage T-cells against CD38-expressing tumor cells and enhance their CD3-triggered activation via CD28 co-stimulation. Here, we performed a preclinical investigation to evaluate the potential therapeutic value of the SAR442257 to treat PTCL, knowing that both CD28 and CD38 might represent targets in tumor T cells.
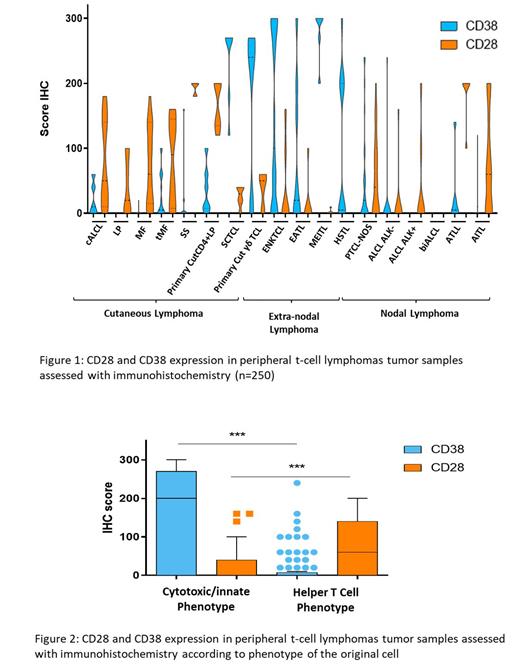
Material and methodsCD28 and CD38 expression was studied in tumor cells of 268 PTCL patients. In situ semi-quantitative evaluation was done using immunohistochemistry on FFPE samples (n=250), and quantitative evaluation was performed using flow cytometry (FCM) on PBMCs (n=18) and PTCL lines (n=6). Multiplex immunofluorescence stainings with tumor cell marker were also performed on biopsies sections of selected cases. In vitro cytotoxic assays were performed by using 24h co-cultures of PTCL cell lines stained with a viability dye with allogeneic blood cells from healthy donors (HD) and SAR442257 or control TriAbs with null mutations in CD28 (D28), CD38 (D38) or both recognition domains at various concentrations (0-25000 pM) at 10:1 effector-to-target ratio. For primary PTCL cells, similar experiments were done with appropriate panels of surface markers aiming to identify tumor cells. Activation assays, measuring expression of CD25 and CD69, were done on PTCL lines and PBMCs from HD using the afore mentioned TriAbs at similar concentrations. ResultsWe found a recurrent but variable expression of CD28 and CD38 among the different PTCL entities (Figure 1). Overall, CD38 expression by tumor cells was found in 42% of PTCL samples in situ and 67% of PBMCs (ranging from 2.5% to 65%; data not shown). CD28 was found in neoplastic T cells in 56% of PTCL samples by IHC and 100% of PBMCs with a strong heterogeneity ranging from 30% to 99.4% (data not shown). CD38 and CD28 showed a range of expression among PTCL categories, with CD38 being more frequently expressed (Avg. IHC score: 250-300)) in PTCL diseases with a cytotoxic profile postulated to derive from innate immune/cytotoxic cells whereas CD28 was more frequently expressed (Avg. IHC score: 150-200) in PTCLs known to derive from helper T-cells (p<0.001, Figure 2). Co-expression of both targets was rare. Multiplex stainings confirmed co-expression of CD38 and/or CD28 targets in tumor cells but consistent with the FCM analysis, CD28 and CD38 were variably expressed in tumor T-cells of TFH PTCLs.
The SAR442257 induced efficient killing of all CD28+/CD38+ and CD28-/CD38+ PTCL lines (with maximum killing reaching about 35%), while the D28 and D38 control TriAbs showed less efficient cytotoxic activities in six different cell lines. SAR442257 also induced CD25 and CD69 expression on normal T-cells suggesting efficient T-cell activation, (data not shown).
Conclusion
Altogether, this study shows that 1) most PTCL cells express at least CD28 or CD38, and 2) SAR442257 can efficiently kill malignant PTCL cells, while ensuring effective T-cell activation; In view of these results, clinical investigation of SAR442257 in PTCL is warranted.
Disclosures
Bisht:Sanofi R&D: Current Employment, Current equity holder in publicly-traded company. Van de Velde:Sanofi: Current Employment, Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal