Background :Under the pandemic of coronavirus disease 2019 (COVID-19), in addition to all trans retinoic acid (ATRA) and oral arsenic(RIF), high-risk acute promyelocytic leukemia (APL) patients might lack of the necessity of intravenous cytotoxic chemotherapy infusions timely. Therefore, there is an urgent need for an oral, efficient and safe regimen for high-risk APL.
Methods :We retrospectively analyzed 66 high-risk APL patients from January 2017 to to July 2022, of which 6 (9.1%, 6/66) patients died in the emergency room without treatment within 24 hours of arriving at our hospital (cerebral hemorrhage in 4 cases and multiple organ failure in 2 cases) and were excluded from the final analysis. A total of 60 patients received anti-leukemia treatment: 22 inpatients receiving oral etoposide (VP16) as cytoreductive chemotherapy from February 2020 to July 2022, and 38 inpatients receiving intravenous cytoreductive chemotherapy as historical control group from January 2017 to December 2019.
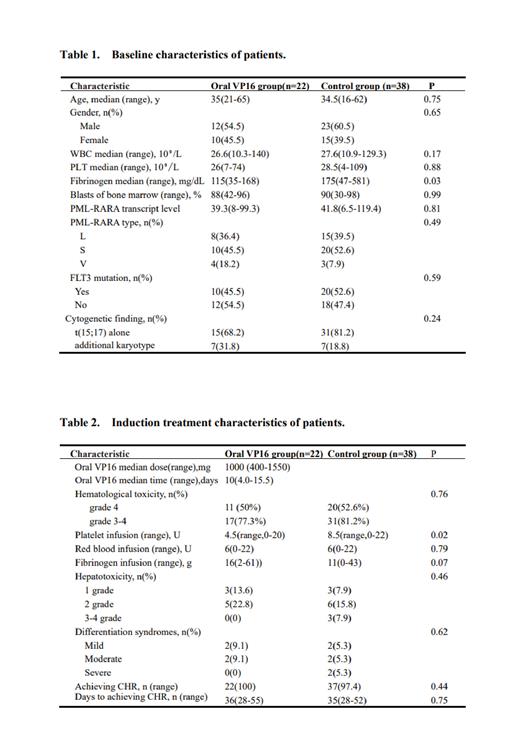
Results :The median age of oral VP16 group and the control group were 35 years (21-65) and 34.5 years (16-62) (P=0.75). The median dose of oral VP16 was 1000mg (range, 400 to 1550). Differentiation syndrome occurred in 4 patients (18.2%) without severe forms in oral VP16 group and 7 patients (18.4%) in intravenous cytoreductive group(P=0.98). Except for one patient died of early death during induction therapy in the control group, all 59 evaluable patients (100%) achieved complete hematological remission(CHR) after induction therapy and complete molecular remission (CMR) after consolidation therapy. The median time to CHR and CMR was 36 days (range, 28-55) vs. 35 days (range, 28-52) (P=0.75) and 3 months (range, 1.1-5.5) vs. 3.3 months (range, 0.9-5.8) (P=0.58) in oral VP16 group and the control group, respectively. The median follow-up time was 56.6 months (range,10.3-76.4). Two (9.1%) patients in oral VP16 group and 3 (7.9%) patients in the control group experienced molecular relapse. The 2-year estimated OS and EFS was 100% vs. 94.7%(P=0.37) and 90.9% vs. 89.5%(P=0.88), respectively.
Conclusions :A completely oral, efficient and safe induction regimen including oral VP16 as cytoreductive chemotherapy combined with ATRA and RIF is more convenient to administer for patients with high-risk APL.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal