Background: Acute myeloid leukemia (AML) with germline DDX41 mutation is implicated in 2-5% of AML patients (pts). Unlike other genetic predispositions in AML, germline DDX41 mutations are associated with later-onset myeloid malignancies (median age 69 years), making it difficult to distinguish clinically from sporadic AML. Data suggests that pts with germline DDX41-mutated AML have more favorable outcomes compared to sporadic AML. However, data regarding optimal treatment and long-term prognosis remains limited.
Methods: Pts treated for AML at The University of Kansas Medical Center between 2015 and 2022 with at least one DDX41 variant were identified via retrospective search. Demographics, disease characteristics, treatments, and vital status were analyzed. DDX41 and other co-occurring mutations included for analysis were pathogenic, likely pathogenic, or variants of uncertain significance. Mutations were considered germline in origin if the variant allele frequency (VAF) exceeded 40% or via search of ClinVar repository in borderline cases (VAF 35-40%, n=2) or if VAF was not reported (n=3). Pts suspected to have germline DDX41 mutations were included in the primary analysis.
Results: We identified 42 pts with AML with at least one DDX41 variant. Two cases had donor-derived leukemia after prior related donor allogeneic stem cell transplant (SCT) and two cases only had somatic DDX41 p.R525H mutations. These were excluded from primary analysis.
36 pts had suspected germline DDX41 mutations and were included for analysis. 28 (78%) had DDX41 mutations at diagnosis and 8 were found later with a broader next-generation sequencing (NGS) panel (in 6, after induction and in 2, after relapse).
Average age of pts with germline DDX41-mutated AML was 67 years, with 3:1 male predominance. Most had preceding cytopenias, and 12 (33%) had antecedent myelodysplastic syndrome (MDS). Family history of malignancy was common (Table 1).
27 (77%) of the available 35 karyotypes at diagnosis were normal. One patient did not have an available karyotype due to lack of records. 28 pts had testing at diagnosis that included DDX41 in the NGS panel. Of these, 57% (n=16) had two DDX41 mutations, with one germline and one somatic mutation. The most common germline DDX41 mutations were p.M1I (14.3%) and p.D140fs*2 (10.7%), and the most common somatic mutation was p.R525H (39.3%). Other commonly concomitant mutations were in ASXL1 (36.1%), CUX1 (22.2%), and KMT2C (13.9%). Mutations such as NPM1 (5.6%), TP53 (5.6%), and FLT3 (2.8%) were uncommon. Germline testing was performed in 5 (13.9%) pts, confirming germline variants.
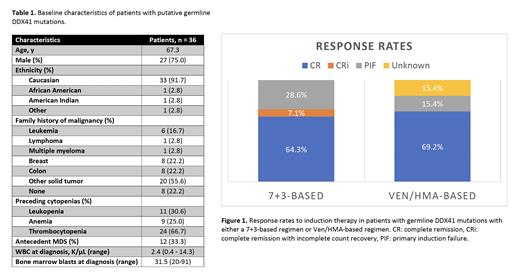
39% (n=14) were treated with a 7+3-based regimen (average age 60 years) with a 71% response rate. 36% (n=13) were treated with venetoclax/hypomethylating agent (Ven/HMA) (average age 72 years) with a 69% response rate (Figure 1). Two pts receiving Ven/HMA died during the first cycle (one from myocardial infarction, one from pneumonia). 18 pts (50%) received SCT, and 50% of those (n=9) had a related donor. 33% of related donors (n=3) were tested for a germline DDX41 mutation prior to transplant.
At last follow-up, 44% were alive in CR (n=16), 5.5% alive with disease (n=2), 14% deceased in CR (n=5), 25% deceased with disease (n=9), and 11% deceased with unknown status (n=4). Median survival was 12.8 months (average 19.6 months in pts with SCT vs. 8.3 in those without, p = 0.012). 11 pts died from AML (33.3%) or complications (22% infection, 6% bleeding).
Two pts with only somatic DDX41 mutations were adverse risk (with ASXL1 mutation) and intermediate risk per 2022 ELN risk stratification, both deceased but in CR at time of death. The adverse-risk patient underwent SCT with unrelated donor (survival 7.3 months). The intermediate-risk patient was re-induced after PIF and died from aortic stenosis (survival 2.2 months).
Conclusions: In our cohort of germline DDX41-mutated AML, responses to a 7+3- or Ven/HMA-based regimen were similar. SCT evaluation remains crucial with improved overall survival in pts receiving SCT. Our study highlights the importance of comprehensive genetic testing that includes DDX41 at diagnosis, along with testing of related donors and children of pts with a germline DDX41 mutation due to the impact of positive testing on donor selection and to identify pts at risk of AML. The presence of only somatic DDX41 mutations was rare, and further research is needed on the impact of somatic DDX41 mutations alone.
Disclosures
Lin:Bio-path Holdings: Consultancy, Research Funding; Astellas Pharma: Consultancy, Research Funding; Celyad: Research Funding; Aptevo Therapeutics: Research Funding; Cleave Biosciences: Research Funding; Ciclomed: Research Funding; Jazz Pharmaceuticals: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal