Background
Venetoclax (Ven) is a potent Bcl-2 inhibitor, approved by FDA for use in combination with hypomethylating agents (HMAs) or low dose cytarabine (LDAC) for newly diagnosed acute myeloid leukemia (AML) in adults 75 years or older, or who have comorbidities based on VIALE trial. The combination regimen often results in severe neutropenia. Febrile neutropenia is one of the most common treatment-associated complications in the management of AML, increasing risk of hospitalization and death. Severe neutropenia is defined as absolute neutrophil count (ANC) <500/uL and often leads to clinicians holding treatment. There have been some studies about racial disparities in genetics and survival outcomes amongst AML patients, however there are limited studies regarding severe neutropenia and its implication between different races. There is also no prediction tool for infection-related death (IRD), overall survival (OS) and event-free survival (EFS) that is helpful in dosing Ven for AML thus far.
Method
We queried Northwell Health Cancer Institute's electronic health records to identify all patients with AML who received at least one cycle of Ven and HMA between January 2019 and December 2022. We retrospectively analyzed 76 patients who had received at least 3 cycles of Ven with HMA. Data on neutropenic duration was collected by counting the number of days patients had an ANC<500/uL starting from cycle 3 until data censor date, change of treatment, relapse, or transplant. OS was calculated as the time between date of definitive diagnosis to death or date of last contact and EFS was calculated as the time between date of diagnosis to events such as stopping Ven due to intolerability, relapse or transplant. We calculated Neutropenic Index for Ven Exposure (NIVE) by dividing the total number of days of severe neutropenia by total number of days on Ven and multiplying by 100 days. Statistical analysis was done by using Stata.
Findings
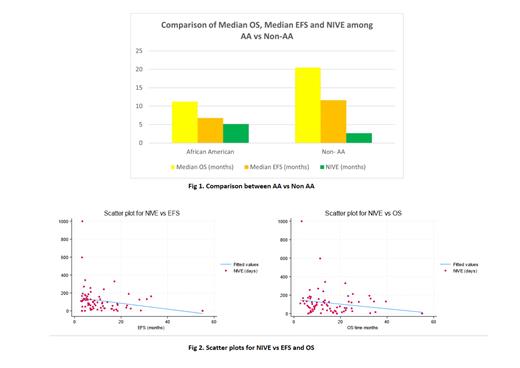
The study cohort consists of 63.1% White, 15.7%, African-American (AA), 7.8% Asians, 2.6% mixed race and 10.5% unknown. While 14% of the patients died of infection, 28.6% of those who died of infection were AA.Among AA, 55% had complex karyotype. Risk of death due to infection was 2.36 times higher in AA compared to non-AA (p= 0.08). We also found that the median OS for non-AA was 20.5 months vs 11.2 months for AA population (p= 0.66) and EFS was 11.6 months for non-AA vs 6.8 months for AA patients (p= 0.25) (Fig.1).
On subgroup analysis, the median of NIVE was 77 in patients who received Ven in the frontline setting vs 109 days in relapsed/refractory setting (p= 0.21).The median NIVE for patients who received Azacytidine+Ven was 61.6 vs 94 days for Decitabine+Ven group (p= 0.12). On univariate analysis, high NIVE is associated with lower EFS (p= 0.04) and shows a trend towards decrease in overall survival (p= 0.09) (Fig.2).
On Mann-Whitney test, NIVE is higher in AA population (p= 0.04), with median NIVE of 157 days in AA patients vs 82 days in non-AA patients. On multivariate regression analysis including age, sex, ELN risk stratification and age at diagnosis, median NIVE is also higher in AA compared to non-AA patients(p= 0.002) . NIVE is also found to be higher in the patient cohort that died of infections over other causes (p= 0.02). On subgroup analysis, patients with NIVE > 60 were found to have a significantly lower median OS than the NIVE ≤ 60 group (p= 0.04).
Discussion s
Our findings suggest that AA patients have significantly lower EFS and higher risk of infection-related death (IRD) compared to non-AA group. This highlights the need for further studies to identify the cause of disparities in IRD and outcomes among AA patients which needs explanation beyond socioeconomic factors and access to treatment.
Our study shows disparities in median NIVE between AA vs non-AA patients. Larger studies are needed to clarify if AA patients are more susceptible to severe neutropenia while on Ven. Racial consideration may be important in deciding the optimum dose of Ven and interval between cycles.
The positive association of NIVE with relapsed/refractory group & Decitabine+Ven group compared to frontline & Azacytidine+Ven group respectively suggests that the former group may benefit from adjustments in Ven dosing or total drug exposure per cycle.
NIVE may be a novel prediction tool for IRD, EFS and possibly OS for AML patients on Ven which may help with patient management.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal