Introduction:
Clonal hematopoiesis (CH) is a pre-malignant state wherein somatic mutations confer a proliferative advantage to hematopoietic stem cells, driving inflammatory changes that increase the risk of age-related diseases and mortality. CH is common in individuals with solid cancers and associated with poorer overall survival due to increased tumor progression, raising the possibility that CH alters the tumor-immune microenvironment (TIME). This study defines the inflammatory program of tumor-infiltrating leukocytes to help elucidate the role of CH in the TIME.
Methods:
Somatic mutations compatible with CH were detected in the whole exome sequences of 6,537 cancer patients with 16 major cancer types from The Cancer Genome Atlas (TCGA) project using Mutect2. CH variant curation followed a pre-defined list of putative variants in 63 genes known to drive hematologic clonality. Calls were filtered to remove sequencing artifacts, germline variants, and small clones with variant allele fraction (VAF) < 2%. Bulk RNA-seq analysis was conducted on data from the colon adenocarcinoma (n=240) and lung adenocarcinoma (n=402) groups. Sequencing reads were pseudo-aligned and quantified with kallisto, then differential expression analysis was performed with DESeq2, controlled for cancer type, tumor biopsy site, and patient sex. Differentially expressed genes were subject to gene set enrichment analysis (GSEA) of gene ontology (GO) terms using clusterProfiler.
Results:
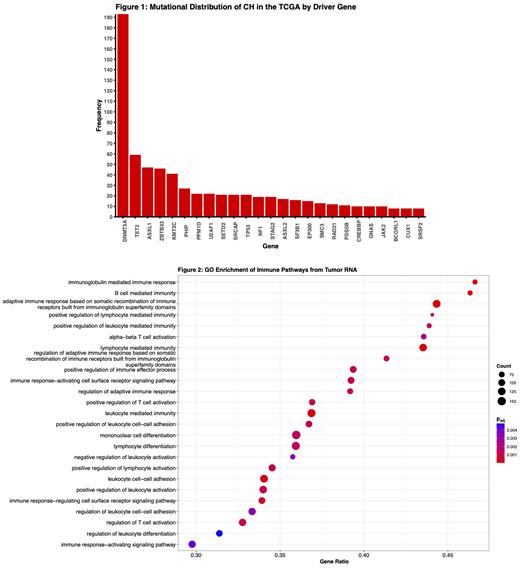
The prevalence of CH in this cohort was 8.8% (n=576/6,537) and its presence was strongly associated with age (p=1.0x10 -25). The highest rate of CH was found in cutaneous melanoma (23.5%, n=110/468) and the lowest rate was in esophageal carcinoma (2.4%, n=3/126). Most patients with CH had just 1 variant (82.8%, n=477/576), but some patients exhibited 2 variants (10.1%, n=58/576), and a small group of patients carried 3 or more variants. A total of 772 CH variants were detected in the cohort, and as expected their distribution across the CH driver genes was not uniform: DNMT3A mutations were most common (n=193), followed by TET2 (n=59), and ASXL1 (n=47; Fig 1). KMT2C mutations were also present in this cohort (n=41) at far higher rates than reported in the general population, providing possible evidence that KMT2C-mutant CH is associated with cancer or its treatments.
Across the group of colon and lung cancer patients subject to RNA-seq analysis, a total of 220 genes (111 upregulated, 109 downregulated) were found to be differentially expressed by CH status at p adj < 0.05. GSEA of GO terms revealed significant activation of gene sets involved in innate and adaptive immune processes with upregulation of both positive and negative regulatory systems, suggesting more intricate mechanisms of immune dysfunction as opposed to generalized hyper-inflammatory activity in the TIME (Fig. 2). GSEA also revealed enrichment of non-immune pathways tied to tumorigenesis. Among the significantly dysregulated genes are the neutrophil activators PPBP and CXCL6, monocyte/macrophage markers LILRA2, CPM, CHI3L1, and CD163L1, and the natural killer cell receptors KLRC2/3. Strikingly, these inflammatory changes were absent in patients with DNMT3A, TET2, or ASXL1 (DTA) CH, and were instead driven by non-DTA CH mutations in a VAF-dependent manner. Investigation of tumor transcriptomes within each cancer type revealed that CH in colon cancer patients has reciprocal effects on inflammation and immunity - DTA CH in this cancer demonstrated suppression of immune-associated gene sets while non-DTA CH was more consistent with the general trend of immune enrichment and activation.
Conclusions:
This work demonstrates that while general inflammatory changes are evident at the tumor level with CH, there is a critical need to recognize the nuances of CH-associated immune mechanisms in cancer, which appear to vary by cancer type and CH driver. Ongoing efforts will continue to characterize the TIME of the other TCGA cancers through transcriptomic analysis and in situ histological imaging. This study also provides some early clinical evidence for the selection of KMT2C variants in cancer patients, though more work is needed to understand their clonal dynamics and clinical implications. In light of these findings, future studies will pave the way for CH as a biomarker in cancer medicine and a cornerstone of precision immuno-oncology.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal