Background: People with hemophilia A (PwHA) experience frequent bleeding into joints, muscles and soft tissues due to a deficiency of factor (F)VIII, which has a substantial negative impact on their health-related quality of life (HRQoL). The American Thrombosis and Hemostasis Network (ATHN) ATHN 7 research study is: A Natural History Cohort Study of the Safety, Effectiveness, and Practice of Treatment for People with Hemophilia (NCT03619863). ATHN 7 prospectively monitors the use of hemophilia A (HA) and B therapies, including emicizumab. The aim of this abstract is to contribute to the literature on disease burden in PwHA. We describe baseline scores for a subset of adult PwHA enrolled in ATHN 7 who were receiving emicizumab and completed the Patient-Reported Outcomes Measurement Information System (PROMIS®) Profile-29 measure at the time of study entry.
Methods: ATHN 7 is a longitudinal, prospective observational cohort study conducted at 26 ATHN-affiliated sites in the United States (US). Clinical and demographic information was collected at baseline. Participants completed patient-reported outcome measures, including PROMIS-29, at baseline. The PROMIS-29 Profile is a disease-agnostic measure capturing data across physical, mental, and social health domains: physical function, fatigue, sleep disturbance, pain intensity, pain interference, depressive symptoms, anxiety, and ability to participate in social roles/activities. PROMIS measures are scored using a standardized T-score metric with a reference population mean of 50 and a standard deviation (SD) of 10, based on the US general population. Higher scores indicate the participant is experiencing more of the concept being measured (positive or negative). No statistical comparisons were performed in this analysis. A mean standardized T-score of 0.5-1.0 SD worse than the reference population mean is defined as mild symptoms/impairment, 1.0-2.0 SD worse than the mean as moderate symptoms/impairment, and ≥2.0 SD worse than the mean as severe symptoms/impairment (HealthMeasures, 2023).
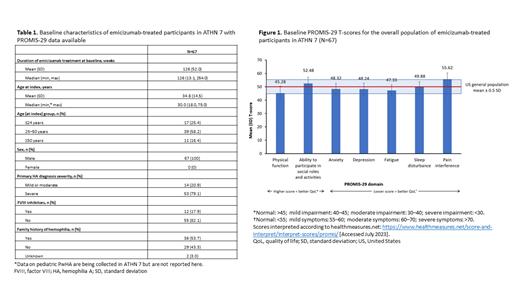
Results: Overall, 67 participants in ATHN 7 receiving emicizumab at study entry completed PROMIS-29 at baseline ( Table 1). Participants had a median (min, max) age of 30.0 (18.0, 75.0) years. Disease severity at diagnosis was mild or moderate for 14 (20.9%) participants and severe for 53 (79.1%) participants. There were 12 (17.9%) PwHA with FVIII inhibitors. At time of study entry, participants had been receiving emicizumab for a median (min, max) of 126 (13.1, 254.0) weeks. Mean PROMIS-29 domain T-scores for the ability to participate in social roles and activities, and symptoms of anxiety, sleep disturbance, depression and fatigue at baseline all fell within 0.5 SD of the mean of the reference population ( Figure 1). PwHA in this analysis did however, demonstrate mild symptoms of pain interference (mean baseline T-score: 55.62; [normal: <55]).
Mean baseline PROMIS-29 domain T-scores were also analyzed according to presence of FVIII inhibitors, disease severity, and age. PwHA with FVIII inhibitors had mild impairment of physical function (42.17 [normal: >45]) and mild symptoms of pain interference (58.64; [normal: <55]), while those without inhibitors had physical function (45.96; [normal: >45]) and pain interference (55.00; [normal <55]) within or on the boundary of 0.5 SD of the reference population. Similarly, people with severe HA without FVIII inhibitors had mild impairment of physical function (44.76; [normal >45]) and mild symptoms of pain interference (55.49; [normal: <55]), while people with mild/moderate HA without inhibitors had physical function (49.85; [normal: >45]) and pain interference (53.46; [normal: <55]) within 0.5 SD of the reference population. Physical function decreased with age, from 51.71 in the group aged ≤24 years (normal: >45) to 36.18 (moderate impairment) in the group aged ≥50 years, while pain interference increased with age, from 51.18 in the group aged ≤24 years (normal: <55) to 59.80 (moderate symptoms) in the group aged ≥50 years.
Conclusions: In this analysis, we observed that HRQoL in PwHA receiving emicizumab, as measured by PROMIS-29 domain T-scores, generally did not differ from that of the US reference population. PwHA did report mild symptoms of pain interference. Results should be interpreted with caution given that no statistical tests were performed, and subgroup numbers are small.
Disclosures
Buckner:Novo Nordisk: Honoraria; Tremeau Pharmaceuticals: Consultancy; BioMarin: Consultancy; CSL Behring: Honoraria; Octapharma: Honoraria. Daoud:American Thrombosis and Hemostasis Network: Current Employment. Lee:Genentech, Inc.: Current Employment, Current equity holder in publicly-traded company. Lim:Genentech, Inc.: Current Employment, Current equity holder in publicly-traded company. Raimundo:Genentech, Inc.: Current Employment; F. Hoffmann-La Roche Ltd.: Current equity holder in publicly-traded company. Recht:Hema Biologics: Consultancy, Research Funding; BioMarin: Research Funding; Grifols: Research Funding; Bayer Pharmaceuticals: Research Funding; Genentech, Inc.: Consultancy, Research Funding; CSL Behring: Consultancy, Research Funding; Pfizer: Consultancy; Sanofi: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; uniQure: Consultancy, Research Funding; LFB: Research Funding; NovoNordisk: Research Funding; Partners in Bleeding Disorders: Membership on an entity's Board of Directors or advisory committees; ATHN: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal