Introduction:
The long-term efficacy of anti-CD19 chimeric antigen receptor (CAR)-T cell in refractory or relapsed (r/r) adult B-cell acute lymphoblastic (B-ALL) patients is limited, and the recurrence rate is high. CAR-T cell depletion and limited CAR-T cell persistence are some of the most common reasons for relapse. Our previous in vitro studies confirmed that dendritic cell (DC) vaccines targeting tumor antigens could induce CAR-T cell rejuvenation and increase the killing function of CAR-T cells. So, we designed a clinical trial to study CD19 CAR-T cell combined with DC vaccination for adult r/r B-ALL to explore whether this therapy improves LFS. (clinicaltrials.gov, no: NCT03291444).
Methods:
Adult r/r B-ALL patients who expressed HLA-A1101, A2402, or A0201 and had high expression of EPS8 or WT1 were eligible. An EPS8 peptide-derived DC (EPS8-DCs) vaccine was used in EPS8-high patients, while a WT1 peptide-derived DC (WT1-DC) vaccine was used in EPS8-negative patients with WT1 positivity. Lymphodepleting chemotherapy comprising fludarabine (30 mg/m²) and cyclophosphamide (300 mg/m²) was administered intravenously daily for 3 days before CD19 CAR-T cells infusion. After 4 weeks of CAR-T infusion, if bone marrow morphologic remission had been achieved, DC vaccination was administered intradermally every 2 weeks for 4 doses.
Results:
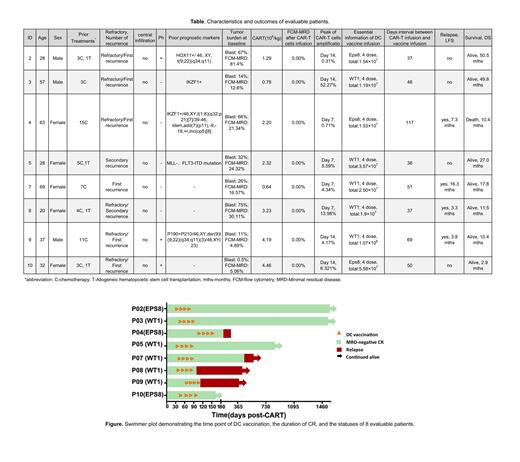
Eight adult patients with r/r B-ALL were enrolled and successfully received CAR-T cells and DC cells, of which 4 (50%) relapsed after allogeneic hematopoietic stem cell transplantation. They were successfully administered one dose of CD19 CAR-T with a median dose of 2.26×10 6/kg (range 6.4×10 5/kg to 4.46×10 6/kg) on day 0 and four doses of DC vaccination with a median dose of 5.44×10 6 (range 2.97×10 6/dose to 2.68×10 7/dose) every 2 weeks after 4 weeks of CAR-T infusion. All eight evaluable patients achieved complete response (CR) after receiving CD19 CAR-T. With a median follow-up of 608 days, the median LFS time was 489 days, and the median OS was not reached. Seven of the eight evaluable patients were still alive. Four (50%) were in continuous MRD-negative remission at the cutoff time, and two of them (pt 02 and pt 03) maintained MRD-negative CR for more than 4 years.
The median peak of CAR-T cell expansion in the PB was detected on day 7 after infusion of CD19 CAR-T. The median persistence time of CAR-T was 336 days (range 84 to 1549 days). CAR-T cells were reamplified after infusion of the DC vaccine. For patients with an LFS of more than 2 years (pt 02, pt 03, and pt 05), CD19 CAR-T cells were still detectable for more than 1 year, with a maximum of 4.2 years in pt 03. The activity of the CTLs measured by IFN-γ ELIspot showed that IFN-γ-secreting CTLs were significantly increased after DC vaccination. These assays showed that antigen-specific cellular immune activity was enhanced after vaccination.
No grade ≥3 cytokine release syndrome (CRS) or immune effector cell-associated neurotoxicity syndrome (ICANS) occurred after infusion of 4sCAR19. No grade ≥3 events occurred during the infusion of the DC vaccine. Only 1 of 8 patients experienced local skin reactions after infusion of the DC vaccine.
Conclusions:
This study reports a novel combination therapy strategy (CAR-T cell combining with individualized DC vaccination) for adult r/r B-ALL. DC vaccination has higher safety, may prolong the persistence of CAR-T cells, and may prolong the survival time and quality of life. CAR-T cell therapy combining with DC vaccination is a potential therapy strategy for adult r/r ALL patients who are not eligible for transplantation or who relapse after transplantation.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal