Background: Despite the efficacy of venetoclax (VEN) in frontline CLL, optimal combination regimens and duration of treatment remain unclear. We hypothesized that cytoreduction with bendamustine/rituximab (BR) induction followed by venetoclax/rituximab (VR) consolidation for a fixed 1-year duration would be associated with an increased rate of undetectable minimal residual disease (uMRD) compared to historical controls and a reduction in the risk of tumor lysis syndrome (TLS). Here we report updated data from a fully-enrolled ongoing phase 2 multicenter, US, single-arm, open-label study (NCT03609593) designed to assess the safety and efficacy of BR-VR in previously untreated CLL patients (pts).
Methods: Previously untreated CLL/SLL pts ≥ 18 years requiring therapy per iwCLL criteria initially received 3 cycles of bendamustine 50-90 mg/m 2 daily for 2 days and rituximab 375 mg/m 2 every 28 days for 3 cycles. Following BR, VEN was initiated with a standard dose escalation from 20 mg to 400 mg daily over 5 weeks. This was followed by 6 cycles of VR with rituximab given monthly and 5 cycles of VEN alone (12 cycles of VEN in total). Additional eligibility included: ECOG PS ≤ 2, hemoglobin ≥8g/dL, ANC ≥1000/mm 3, and platelets ≥50,000/mm 3. Response was assessed by 2018 iwCLL criteria with uMRD testing by central flow cytometry at a level of <10 -4 in peripheral blood (PB) and bone marrow (BM). The primary endpoint was objective response rate (ORR). Secondary endpoints included uMRD rate, time to uMRD, and adverse events (AEs) assessed by CTCAE v 5.0.
Results: As of data cutoff on 15 July 2023, 42 pts were accrued. Baseline demographics were as follows: male/female (29/13), median age 61.5 yrs (range 38-84). Baseline prognostic studies showed unmutated IGHV in 22 (52%) pts, TP53 aberrant (either del(17p) and/or TP53 mutation) in 2 (5%) pt, del(11q) in 3 (7%) pts, and complex karyotype in 11 (26%) pts. TLS risk among 24 evaluable pts at baseline was high (H) in 9 (21%), medium (M) in 22 (52%), and low (L) in 6 (26%).
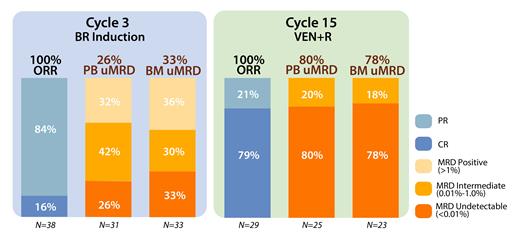
At a median follow-up of 16.6 mo. (range, 3-40), 37 pts remain on study. Of 29 pts with at least 15 mo. follow-up (completing all therapy), the ORR was 100% (79% CR/CRi, 21% PR [due to small residual nodes]). 3 pts died on study (2 due to COVID-19 and 1 developed newly metastatic squamous cell carcinoma and taken off study after achieving a CR post-VEN ramp-up). 1 pt was lost to follow-up while in a CR. Bendamustine was administered at doses of 50 mg/m 2 in 11%, 70 mg/m 2 in 13%, and 90mg/m 2 in 76% of pts. In 38 evaluable pts, response assessments after cytoreduction with BR demonstrated 16% of pts achieved CR/CRi and 84% achieved PR. For evaluable pts at 16 mo., uMRD (<0.01%) in the PB and BM was observed in 80% (20/25) and 78% (18/23) of pts, respectively. MRD was intermediate (0.01% - <1.0%) in 17% (4 patients) in BM (Figure 1 ORR and MRD). Median time to uMRD was 11 mo. (range 3-15) in PB and 10 mo. (range 2.6-15) in BM.
The most common treatment-emergent AEs during BR induction were (any grade/grade ≥3) anemia in 15/4 (36%/10%) pts, nausea in 9/0 (21%/0%), neutropenia in 12/8 (29%/19%), rash in 12/1 (29%/2%), constipation 7/0 (17%/0%), and infusion reactions in 7/1 (17%/2%). 2 pts (5%) developed febrile neutropenia during BR. Emergent AEs during VEN treatment included neutropenia in 21/13 (50%/31%), diarrhea in 18/0 (43%/0%) pts, leukopenia in 14/4 (24%/10%), and nausea in 11/0 (26%/0%).
TLS risk was substantially reduced after BR lead-in. Of 9 H-risk pts at baseline, 1 remained H-risk after BR; of 16 M-risk pts, 5 remained M-risk, with the remainder at L-risk (89% reduction in H-risk TLS).
Conclusions: BR-VR is a safe and well-tolerated regimen in untreated CLL pts. BR debulking substantially reduces TLS risk, and this sequential strategy achieves high rates of PB and BM uMRD across all prognostic risk groups.
OffLabel Disclosure:
Lipsky:AstraZeneca: Consultancy; AbbVie: Consultancy; Synthekine: Consultancy; Beigene: Consultancy. Hill:Bristol Myers Squibb: Consultancy; Genentech: Consultancy, Other: Advisory board, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Other: travel support, Research Funding; Pharmacyclics: Consultancy, Other: Advisory board, Research Funding; Gilead: Other: Advisory board; Incyte: Consultancy; BeiGene: Consultancy; AstraZeneca: Consultancy; AbbVie: Consultancy, Other: Advisory board, Research Funding. Winter:Seattle Genetics: Consultancy; Janssen: Consultancy; AstraZeneca: Consultancy; ADC Therapeutics: Consultancy; BeiGene: Consultancy. Viny:Arima Genomics: Membership on an entity's Board of Directors or advisory committees. Jurcic:Sumitomo Pharma: Research Funding; Blueprint Medicines: Research Funding; Forma Therapeutics: Research Funding; Gilead/Forty Seven: Research Funding; Ionis Pharmaceuticals: Research Funding; Seattle Genetics: Research Funding; AbbVie: Research Funding; Rigel Pharmaceuticals: Consultancy; Syros Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy, Research Funding. Lamanna:Adaptive Biotechnologies: Consultancy; Eli Lilly/Loxo: Research Funding; Pharmacyclics: Consultancy; MingSight: Research Funding; Oncternal: Research Funding; Octapharma: Research Funding; Janssen: Consultancy; Genentech: Consultancy, Research Funding; BeiGene: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; TG Therapeutics: Research Funding.
All Drugs are approved in frontline CLL, though the combination is novel.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal