Background and Significance: MALT1 (Mucosa-associated lymphoid tissue lymphoma translocation protein 1) is a component of the MALT1-BCL10-CARD11 complex downstream from the Bruton Tyrosine Kinase (BTK) on the B-cell receptor signaling pathway. MALT1 is a key mediator of nuclear factor kappa B (NF-κB) signaling, which is the main driver of a subset of B-cell lymphomas. MALT1 is considered a potential therapeutic target for several subtypes of non-Hodgkin B-cell lymphomas and chronic lymphocytic leukemia (CLL), including tumors with acquired BTK inhibitor (BTKi) resistance. Constitutive activation of NF-κB is a molecular hallmark of activated B cell-like diffuse large B cell lymphoma (ABC-DLBCL), and MALT1 may have utility as a treatment option for ABC-DLBCL. Furthermore, a MALT1 inhibitor (JNJ-67856633) showed efficacy in mature B cell malignancies from phase 1 studies (ref 1, 2).
SGR-1505 is an oral potent small molecule allosteric inhibitor of MALT1 that inhibits MALT1 enzymatic activity and demonstrates anti-proliferative activity in BTKi-sensitive (OCI-LY10) and BTKi-resistant (OCI-LY3) ABC-DLBCL cell lines. SGR-1505 administered as a single agent and in combination with the approved Bruton's tyrosine kinase (BTK) inhibitor, ibrutinib, demonstrates tumorostatic and regressive antitumor activity in ABC-DLBCL cell line-derived and patient-derived xenograft models. These data suggest that SGR-1505-mediated MALT1 inhibition may expand therapeutic options for patients with selected B-cell lymphomas, supporting further evaluation of SGR-1505 in clinical trials.
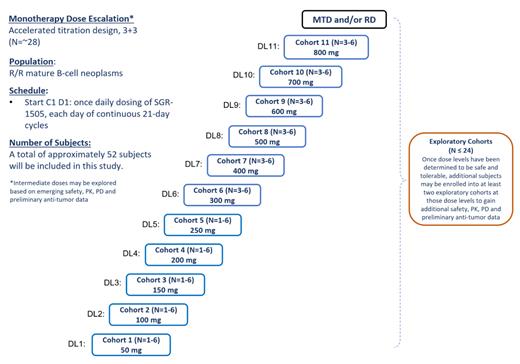
Study Design and Methods: SGR-1505-101 (NCT05544019) is a phase 1, multicenter trial of SGR-1505 as monotherapy in subjects with mature B-cell malignancies (figure 1). At present, the study is open to accrual at multiple investigative sites in the US with plans to expand to Europe. The primary objective is to evaluate safety and tolerability of SGR-1505 as monotherapy and to identify the maximum tolerated dose (MTD) and/or recommended dose (RD). Secondary objectives are to evaluate the pharmacokinetic (PK) profile, food effect, drug-drug interaction, and preliminary anti-tumor activity of SGR-1505. Key inclusion criteria are: history of mature B-cell malignancy (including aggressive and indolent B-cell lymphomas, Waldenström macroglobulinemia, and CLL); measurable or detectable disease according to the applicable disease-specific classification system (Lugano, iwCLL, WWM6); Eastern Cooperative Oncology Group (ECOG) performance status of ≤ 2. Patients with indolent B-cell lymphomas or CLL must have an indication for treatment and not require immediate cytoreductive therapy. Subjects with symptomatic or active CNS involvement, and other conditions or laboratory findings placing them at increased risk to the use of an investigational drug are excluded.
SGR-1505 is initially dose-escalated using an accelerated titration design in cohorts of 1-6 subjects, and at higher dose levels using a conventional 3+3 design.
Disclosures
Yoo:Schrödinger: Current Employment. Tan:Schrödinger: Current Employment. Gupta:Schrödinger: Current Employment. Schuck:Schrödinger: Current Employment. Nie:Schrödinger: Current Employment. Krilov:Schrödinger: Current Employment. Wright:Schrödinger: Current Employment. Weiss:Schrödinger: Current Employment. Lachowicz:Schrödinger: Current Employment. Akinsanya:Schrödinger: Current Employment. Yin:Schrödinger: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal