Introduction:
Cutaneous T-cell lymphoma (CTCL) is a rare form of T-cell lymphoma involving the skin in early stage, and blood, lymph nodes, and viscera in more advanced stages. Mycosis fungoides (MF) and Sezary syndrome (SS) are the most common subtypes of CTCL. Patients (pts) with advanced stage (AS)-MF and SS can have severe symptoms including erythroderma, pruritus, desquamation, and have a poor quality of life.
Extracorporeal Photopheresis (ECP) was approved by FDA for CTCL in 1988. It is the recommended frontline therapy for SS and is also often used in AS-MF. ECP works by immunomodulatory mechanisms and has minimum side effects. However updated data is lacking on the efficacy of contemporary ECP. Most of the studies were published years ago and did not stage pts and evaluate responses using the current criteria. Blood response was not evaluated in most studies. Besides, ECP techniques including the administration of methoxsalen have changed. Seeking to understand the efficacy of ECP in current practice, we conducted a retrospective study including CTCL pts treated with ECP at Moffitt Cancer Center (MCC), FL, USA in the past 13 years.
Methods:
Pts with MF or SS who started ECP at MCC after Jan. 2010 were included. In our practice, pts are typically started on ECP alone. A systemic therapy is added in ~ 3 months when a pt does not respond. Response in blood and skin were evaluated every 3 months.
Results:
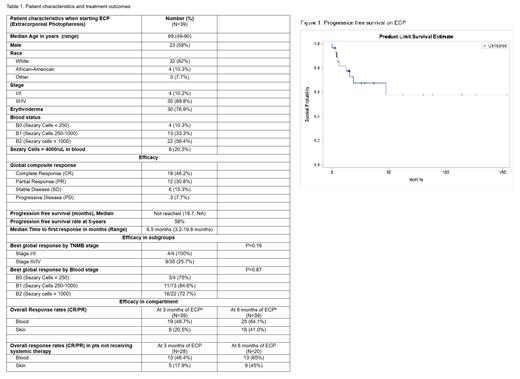
A total of 39 pts were included. 23 (59%) were male; 31 (82%) were Caucasian, 4 (11%) were African-American. When starting ECP, 33 (89%) had stage III/IV disease; 30 (77%) had erythroderma; 10.3%, 33.3% and 56.4% of pts had B0, B1 and B2 disease, respectively (Table 1).
18 (46.2%) pts achieved a best global response of complete response (CR), and 12 (30.8%) achieved partial response (PR). The median time to first global response was 6.5 months (range 3.2-19.8 months).
At 3 months 28 pts were on ECP alone and 11 were on concurrent systemic therapy including: Interferon (4), retinoid acid (3), romidepsin (3), and pralatrexate (1). At 6 months 20 pts were on ECP alone and 19 were on concurrent systemic therapy including interferon (7), retinoid acid (4), Romidepsin (4), and 1 patient for each of mogamulizumab, vorinostat, methotrexate and interferon + retinoid acid.
When evaluated at 3 months, the overall response rate (ORR) in skin was 8/39 (20.5%) in the entire cohort, and 5/28 (17.9%) in pts not on concurrent systemic treatment. When evaluate at 6 months the skin response rates were 16 (41.0%) and 9 (45.0%) in pts with and without concurrent systemic treatment, respectively. The ORR in blood at 3 months was 19/39 (48.7%) in the entire cohort and 13/28 (46.4%) in pts on ECP alone; at 6 months, the ORRs in blood were 25/39 (64.1%) and 13/20 (65%), respectively.
With a median followup of 43 months, the median PFS was not reached (NR). The 5-year PFS rate was 58%. At the last followup, 7 pts who were on ECP alone were in CR, and 3 in PR.
The response rates at 3 months, 6 months and the best response rates were not associated with baseline characteristics including blood stage (B0 vs B1 vs B2), global stage (I/II vs III/IV), and concurrent systemic therapy (p>0.1 for all, table 1, part of the data was not shown).
Discussion:
Our results showed the contemporary ECP therapy had excellent efficacy in SS and AS-MF (ORR 77%). Many pts achieved durable remission (5-year PFS 58%). However, it took a median of 6.5 months to achieve an objective response. Pts with AS-MF and SS have significant symptoms and poor quality of life. Research is needed to identify treatment combinations with ECP to induce a quicker response.
One limitation of our study is, some pts received systemic therapy concurrently with ECP, thus making it difficult to isolate the treatment effect of ECP. As a sensitivity analysis, we evaluated responses of pts on ECP alone in the first 6 months, and in this way decreased the confounding effect. Our study is also limited by the retrospective nature and the limited sample size. Future prospective studies in larger scale are needed to confirm our results.
Disclosures
Dong:EUSA Pharma, a Recordati Group company.: Research Funding; Robert A. Winn Diversity in Clinical Trials Career Development Award, funded by Gilead Sciences: Research Funding. Liu:BioLineRx: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal