Background:
Immune checkpoint inhibitor therapy (ICI) has been variably associated with increased risk of VTE, with some reports suggesting increased risk and others suggesting risk is not different from the baseline venous thromboembolism (VTE) risk in unselected ambulatory patients with cancer.
Methods:
Patients treated with ICI at our institution between 2009 and 2022 were retrospectively identified. Standard baseline characteristics (age, sex, race/ethnicity, BMI), cancer type and stage, type of ICI, KS risk score variables not already captured including white cell count, hemoglobin, and platelet count at time of initiating ICI therapy, as well as other patient and treatment characteristics were collected. Those with history of VTE prior to start of ICI were removed from the analysis population; anticoagulation after starting ICI was a time dependent factor. Cumulative incidence of VTE with death as a competing risk was determined using Fine and Gray technique. Univariate analysis for baseline characteristics was completed. Characteristics that were statistically significant at the 0.1-level or less were carried forward as candidate predictors in multivariable, competing risks models of VTE. Results are presented as hazard ratios with 95% confidence intervals adjusted for multiple comparisons against a reference group.
Results:
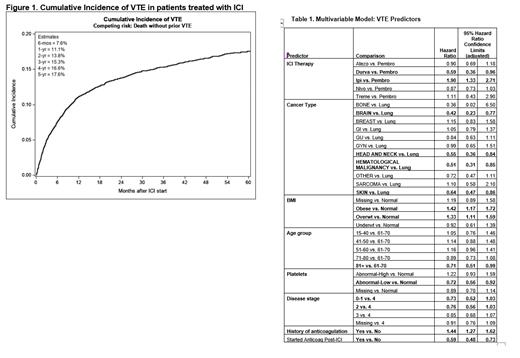
Of 12,007 patients starting ICI therapy, 10,638 were analyzed with median follow-up of 25.1 months. The majority of tumor types included lung (30.3%), skin (15.8%; melanoma, squamous cell), gastrointestinal (11.7%), and genitourinary (11.6%). The most frequently used ICI agents were the anti-PD-1 monoclonal antibodies pembrolizumab (49.7%) and nivolumab (31.8%). The overall cumulative incidence estimates of VTE are summarized in Figure 1. The estimate at six months was 7.6% (95% CI: 7.1 to 8.1%); at one year: 11.1% (95% CI: 10.5 to 11.8%); and at two years 13.8% (95% CI: 13.0 to 14.5%). Among the 1,328 patients who developed VTE, the median time after starting ICI therapy was 5.4 months [IQR: 1.8 to 12.0 months]. Univariate comparison findings carried forward as candidate predictors in the multivariable analyses included sex, age, BMI, race, history of prior anticoagulant therapy (before ICI start), cancer type, disease stage, ICI therapy (by drug), KS, and platelets. The first multivariable model using all predictors identified gender, Khorana risk score, and race as no longer significant. The second model examined the inter-relationships between remaining predictors and the incidence of VTE; as the KS is partially comprised of cancer type, BMI, and platelets, having these as separate predictors removed the influence of the Khorana score since they would provide similar information. Predictors of VTE identified in this model included type of ICI with the use of ipilumumab associated with the highest risk with HR of 1.90 (95% CI: 1.33-2.71) and durvalumab the lowest HR 0.59 (95% CI:0.36-0.96), when compared with pembrolizumab. Type of cancer, stage of cancer, BMI, and high platelet count were also predictive. Starting anticoagulation at the time of or after beginning ICI but not for treatment of VTE was associated with approximately a 40% reduced hazard of VTE compared to patients who had not yet or never started anticoagulation. (HR: 0.59, 95% adj. CI: 0.48 to 0.73). (Table 1)
Conclusion
In this large sample of patients, the use of ICI in VTE naïve patients was associated with an increased risk of VTE, with a cumulative incidence of 7.6% at 6 months compared with a baseline 3-4% risk in general ambulatory patients with cancer. Type of ICI treatment was associated with VTE risk, as was stage of cancer. The individual KS risk predictors of cancer type and BMI were predictive but only elevated platelet count was associated with increased VTE risk-hemoglobin and white cell count were not. Importantly, those that started anticoagulation for reasons other than development of VTE had a significant reduction in VTE risk, suggesting that anticoagulant prophylaxis may be indicated in those patients with multiple risk factors in the setting of ICI treatment. Further analysis of these data will help identify at risk patients starting treatment with ICI who will benefit from VTE prophylaxis.
Disclosures
Connors:CSL Behring: Research Funding; Abbott, Anthos, Alnylam, Bristol-Myers Squibb, Five Prime Therapeutics, Pfizer, Roche, Sanofi, Werfen: Consultancy, Other: Scientific advisory boards. Sussman:Pfizer: Honoraria. Hodi:Bioentre: Honoraria; Immunocore: Honoraria; Catalym: Honoraria; Surface: Honoraria; Novartis: Honoraria; Apricity: Honoraria; Gossamer: Honoraria; Solu Therapeutics: Honoraria; Iovance: Honoraria; Merck: Honoraria; Pieris: Consultancy; Bicara: Honoraria; Astra Zeneca: Honoraria; Curis: Honoraria; Bristol Myers Squibb: Honoraria, Research Funding; Kairos: Honoraria; Rheos: Honoraria; Compass Therapeutics: Honoraria; Checkpoint Therapeutics: Honoraria; Zumotor: Honoraria; Corner Therapeutics: Honoraria; Puretech: Honoraria; Genetech/Roche: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal