Introduction:
Primary cold agglutinin disease (CAD) is an autoimmune hemolytic anemia characterized by the presence of cold-reactive antibodies that lead to red blood cell destruction at low temperatures. Patients with CAD who are transfusion-dependent experience the burden of increased health resource utilization and reduced quality-of-life. The standard-of-care (SOC) in patients with primary CAD has included chemoimmunotherapy, transfusion support, and cold avoidance. Sutimlimab is a humanized monoclonal antibody that selectively inhibits C1-mediated hemolysis. It is the only FDA-approved therapeutic for patients with cold agglutinin disease regardless of history of transfusion, with approval based on two phase 3 clinical studies. The cost-effectiveness of this expensive therapeutic in the care of patients with either transfusion-independent or transfusion-dependent primary CAD is not known. We sought to fill this gap by determining the cost-effectiveness of sutimlimab versus standard-of-care for the transfusion-dependent patient population with primary CAD.
Methods:
For this independent analysis free of industry influence, we built a Markov state transition model of adult patients with transfusion-dependent primary CAD to determine the cost-effectiveness of sutimlimab versus SOC, over a lifetime time-horizon and across accepted willingness-to-pay thresholds in US dollars per quality-adjusted life-year (QALY). For transition probabilities, we employed phase 3 study data (NCT03347396) with descriptive statistics informing the documented pre- versus on-sutimlimab clinical benefit, including the decrement in hemolytic anemia severity and transfusion burden. For SOC, we assumed that all patients undergo the costs and risks of chemoimmunotherapy with bendamustine-rituximab, followed by rituximab monotherapy and subsequent treatment with mycophenolate mofetil, with all patients remaining dependent on transfusion care. Health resource utilization costs for SOC were further supplemented with country- and disease-specific real-world costs over a median of 42.8 and 33.0 months of follow-up after incident primary CAD diagnosis across two claims databases. These costs were assessed from the highest of 1) annualized cost quartile and 2) the worst ordinal category of hemolytic anemia severity (defined as hemoglobin <8 g/dL) across all of 1) hospitalizations, 2) emergency visits, 3) outpatient services, and 4) blood transfusions. We assumed no loss of sutimlimab efficacy and no discontinuation off sutimlimab due to adverse events. Age- and sex-adjusted, disease-specific mortality was employed. Effectiveness was calculated in QALYs and was informed directly from trial-specific utilities. The primary outcome was the incremental cost-effectiveness ratio (ICER) or the incremental net monetary benefit (iNMB), if the intervention was found to be cost-saving. The secondary outcome was a scenario analysis for patients with a body weight of <75 kilograms that mandate lower dosing per-kilogram sutimlimab dosing, as per the FDA package insert. We concluded by conducting deterministic and probabilistic sensitivity analyses, capturing uncertainty in all parameters simultaneously over 10,000 Monte Carlo simulations.
Results:
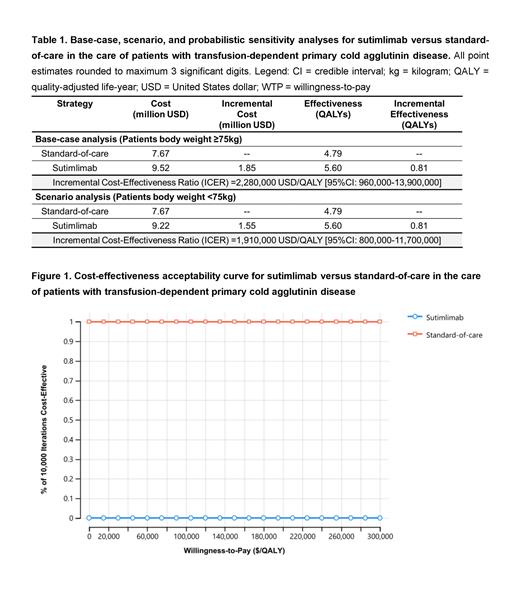
In the base-case analysis, treatment with sutimlimab versus SOC accrued 5.60 and 4.79 QALYs at costs of $9.52 and $7.67 million, respectively, with the ICER for sutimlimab treatment being $2,280,000/QALY [95% credible interval 960,000-13,900,000]. In the scenario analysis for patients with lower body weight (i.e., lower sutimlimab dosing), the ICER was $1,910,000/QALY. Model results were sensitive to sutimlimab cost. In a probabilistic sensitivity analysis, SOC was favored over sutimlimab in 100% of 10,000 iterations. An exploratory threshold analysis showed that the mortality hazard with sutimlimab would need to decrease by 201% for sutimlimab to become cost-effective.
Conclusion:
Sutimlimab is conventionally cost-ineffective compared to SOC in transfusion-dependent patients with CAD. Distributional cost-effectiveness analysis (DCEA) to potentially justify sutimlimab as an equitable therapeutic option despite cost-ineffectiveness in the United States context is needed.
Disclosures
Cuker:New York Blood Center: Consultancy; Synergy: Consultancy; MingSight: Consultancy; UpToDate: Patents & Royalties.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal