Background
Acute lymphoblastic leukemia (ALL) is the second most common acute leukemia in adults. Unfortunately, in Ecuador there are no survival studies in patients ≥15 years with ALL, thus, this is the first national report. The aim of the study was to describe clinical-demographic characteristics and overall survival (OS).
Methods
Medical records of 628 patients from 8 reference hospitals in Ecuador with acute lymphoblastic leukemia (ALL) aged ≥15 years, diagnosed between January/2015 and December/2022, were retrospectively reviewed. A high-risk group was defined as: age ≥ 35 years ; ≥ 30,000/mm3 leukocytes in B-cell acute lymphoblastic leukemia (B-ALL) or ≥ 100,000/mm3 leukocytes in T-cell acute lymphoblastic leukemia (ALL- T) ; CNS infiltration at diagnosis, and/or if EMR was not reached (<0.01%) negative at the end of induction. If none of these factors were present, it was classified as standard risk. OS and treatment-related mortality were evaluated. Before carrying out this study, the approval of a national research ethics committee was obtained, as well as the approval of the 8 participating centers.
Results
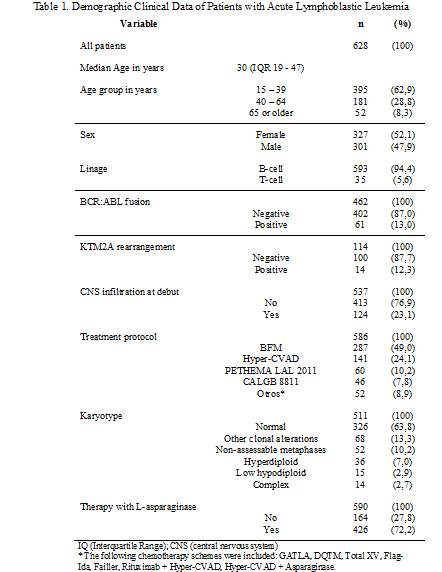
628 patients with ALL were included in the study. Summary of clinical and demographic data Table 1. In this cohort of patients, 9 different regimens of intensive chemotherapy were used. 454/517 (87.8%) patients with B-ALL were considered high risk. 595/628 (94.5%) patients received intensive chemotherapy. 150/595 (25.2%) and 54/595 (9.0%) patients died during the induction and consolidation phase respectively, reaching a mortality related to the treatment of 34.2%. In 290/401 (72.3%) with B-ALL achieved a complete response (CR) after induction, also 199/401 (49.1%) patients achieved minimal residual disease (MRD) negative (<0.01%). 16/235 (6.8%) patients with B-ALL considered high risk who achieved CR received allogeneic hematopoietic stem cell transplantation (HSCT). 32/35 (91.4%) patients with T-cell acute lymphoblastic leukemia (T-ALL) received intensive chemotherapy, of which 17/24 (70.8%) achieved CR, MRD negative (<0.01%), in 8/20 (40.0%) and 3/17 (17.6%) who achieved CR received HCT.
The median OS was 12 months (95% CI 9.8-14.1) with a 5-year OS of 25.6%. The median OS for intensive chemotherapy patients with B-ALL was 12 months (95% CI 9.8 - 14.1) with a 5-year OS of 25.8% and 8 months (95% CI 5.9 - 10.0) with a 5-year OS of 23.5% for T-ALL. The 5-year OS for patients with HSCT was 78.4% (median survival not reached). The median OS for patients with B-ALL who received intensive high-risk chemotherapy was 12 months (95% CI 9.7-14.2) while median survival was not reached in the standard-risk group (p=<0.01). Figure 1. In patients with ALL-B, regimens with or without L-asparaginase achieved a 5-year OS of 26.4% vs 22.9% (p=0.588), respectively. In multivariate analysis for patients with ALL-B on intensive chemotherapy, age [HR 1.02 (95% CI 1.00 - 1.04) p=0.014] was significantly associated with OS, as well as those patients who did not achieve negative an MRD [HR 1.82 (95% CI 1.23-2.69) p=<0.01]. Otherwise, the adolescent and young adults (AYA) group, BCR:ABL fusion, CNS infiltration and leukocyte number at debut, L-asparaginase chemotherapy regimens, and being classified as high-risk group did not show statistical differences associated with OS .
Conclusion
The reported OS rates in this cohort are considerably low when compared to with international available data, being mainly negatively affected by the high treatment-related mortality rate, and the small number of patients who reach a HSCT.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal