Introduction: Several previous studies have indicated that both erythropoiesis-stimulating agents (ESAs) and blood transfusions elevate the risk of venous thrombosis in cancer patients. Nevertheless, there has been inconsistency in the evidence regarding whether ESAs or blood transfusions are more closely linked to thrombotic complications. To address this, nationwide population data was analyzed to explore the association between the use of ESAs, blood transfusions, and the risk of venous thrombosis in cancer patients.
Methods: This study utilized a case-crossover design and analyzed the National Health Insurance Database of Korea. A total of 710,910 patients diagnosed cancer (ICD-10 code: C00.x-C26.x, C30.x-C34.x, C37.x-C41.x, C45.x-C58.x, C60.x-C76.x, C81.x-C85.x, C88.3, C88.7, C88.9, C90.0, C90.1, C91.x-C93.x, C94.0-C94.3, C94.5, C94.7, C95.x, C96.x) between January 1, 2011, and December 31, 2020 were included. Of these, 57,256 (8.03%) identified with venous thrombosis (ICD-10 code: I80.x). The hazard period, starting from the week of thrombosis and going back 12 weeks, was compared to an earlier 12-week control period separated by a 24-week gap. Secondary analyses were conducted with different durations for the hazard/control periods (8 and 4 weeks) to assess the robustness of the results. Patients served as their own controls, and time-dependent covariates such as granulocyte colony-stimulating factor and chemotherapy were evaluated for their association with thrombotic risk. Patients were categorized based on occurrences of inpatient hospitalization, red blood cell transfusion, platelet transfusion, central venous catheter placement, and major surgery during hazard and control periods, and these variables were compared using χ2 tests. Finally, conditional logistical regression was employed to compare the odds of exposure to ESAs, blood transfusions, and other thrombosis risk factors during the hazard period to the exposure odds during the control period.
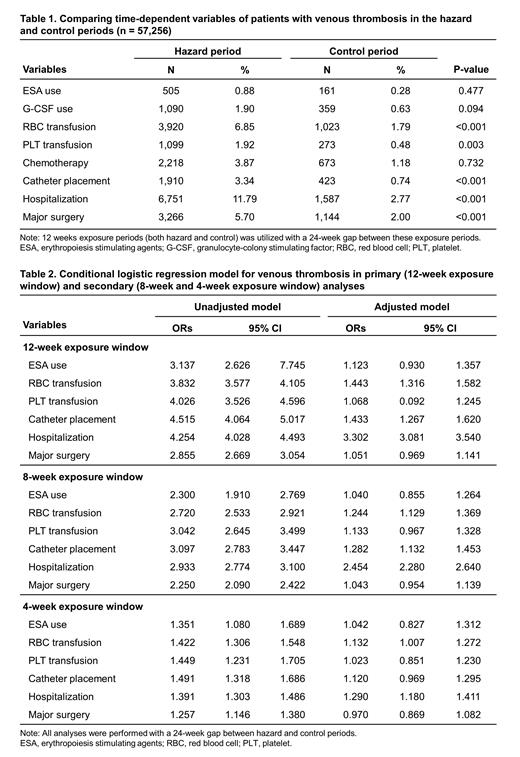
Results: There were similar rates of ESA use during the hazard period and the control period (0.88% vs. 0.28%; P = 0.477). However, red blood cell transfusions (6.85% vs. 1.79%; P <0.001), platelet transfusions (1.92% vs. 0.48%; P = 0.003), insertion of a central venous catheter (3.34% vs. 0.74%; P <0.001), hospitalizations (11.79% vs. 2.77%; P <0.001), and major surgery (5.70% vs. 2.00%; P <0.001) differed significantly between the two exposure periods. All of these factors were more frequent during the hazard period compared to the control period. In the crude conditional logistical regression model, the odds ratio (OR) for ESA use was significantly increased (OR = 3.14; 95% CI: 2.63, 7.75). However, after controlling for other covariates including red blood cell transfusions, platelet transfusions, central venous catheter insertion, hospitalization, and major surgery in an adjusted model, ESA use was found to be non-significant (OR = 1.12; 95% CI: 0.93, 1.36). In contrast to ESA use, red blood cell transfusion was associated with an increased risk of thrombosis in both the crude model (OR = 3.83; 95% CI: 3.58, 4.11) and the adjusted model (OR = 1.44; 95% CI: 1.32, 1.58). This study also investigated whether varying the lengths of the hazard and control periods (8 and 4 weeks) would affect the association between ESA use, red blood cell transfusions, and thrombotic events. The results showed that the association remained consistent regardless of the duration of the periods. Overall, these findings suggest that while there was an increased risk of thrombosis associated with red blood cell transfusions, the use of ESAs did not show a significant association with thrombotic events after adjusting for other relevant factors.
Conclusions: This study revealed a stronger association between red blood cell transfusions and thrombotic complications in cancer patients. These findings suggest that red blood cell transfusions may have a higher thrombotic risk compared to ESAs in this particular patient population. Furthermore, the results indicate that the use of ESAs in cancer patients might not increase the risk of thrombotic complications depending on the exposure duration. Further studies are needed to assess the impact of ESAs and blood transfusions on thrombotic risk in different populations.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal