Introduction:
Sickle Cell Disease (SCD) is an inherited hemolytic disorder due to an autosomal recessive mutation affecting mainly African Americans. SCD can lead to various complications, including Sickle Cell Crisis (SCC), Acute Chest Syndrome (ACS), strokes, and different osseous complications. Notably, these complications can be provoked using steroids. Sarcoidosis is a multisystem inflammatory disorder that is characterized by the presence of bilateral hilar lymphadenopathy that is commonly treated with steroids. Unfortunately, it is also preferentially affecting African Americans. The complication rates in patients with both diseases have rarely been described. We aim to describe the effect of sarcoidosis on the mortality and complication rates of admitted patients with SCC.
Methods:
Using the National Inpatient Sample (NIS) 2016-2020, we analyzed adult SCC and ACS hospitalizations with and without sarcoidosis using International Classification of Diseases - 10 Clinical Modification (ICD-10-CM) codes. The primary outcome was inpatient mortality. Secondary outcomes were inpatient morbidities, mean length of stay (LOS), and mean total hospital charge (THC). A multivariate logistic regression and linear regression analyses were used to adjust for potential confounders.
Results:
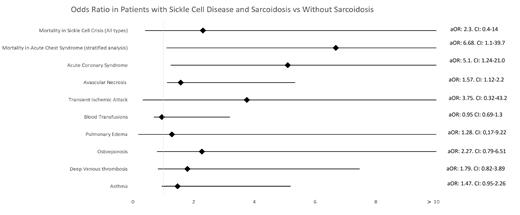
Out of 359655 patients hospitalized with SCC, only 4.6% had concomitant sarcoidosis. Of these, 70% were females, with a mean age of 38.4 compared to an average age of 32 in patients without sarcoidosis (p-value <0.05). The mean LOS for patients with sarcoidosis and SCC was 6.4 days, while it was 5.1 days for patients without sarcoidosis (p-value 0.001). While adjusting for common comorbidities and patients' characteristics, patients with SCC and sarcoidosis did not have a statistical mortality difference compared to patients without sarcoidosis [adjusted odds ratio (aOR): 2.3, CI: 0.4-14). On stratified analysis, patients with ACS and sarcoidosis had increased odds of mortality with an aOR of 6.68, CI: 1.1-39.7, and a p-value of 0.037. Moreover, amongst patients who had SCC, patients with concomitant sarcoidosis had a higher risk of acute coronary syndrome (aOR: 5.1, CI 1.24-21.0) and avascular necrosis (aOR: 1.57, CI: 1.12-2.20). There were no statistically significant differences in the odds of ischemic strokes, transient ischemic attacks, acute and chronic kidney disease, risk of intubation, pulmonary edema, osteoporosis, venous thrombosis, and THC. Figure 1 shows the Forrest plot for multivariate analysis of in-hospital morbidities when adjusted for patient demographics, comorbidities, and hospital characteristics.
Conclusion:
Patients admitted with ACS and sarcoidosis had higher odds of mortality. Patients with SCC and sarcoidosis also had higher odds of acute coronary syndrome, avascular necrosis, and mean LOS. Nevertheless, this has not impacted other inpatient comorbidity.
Disclosures
Liles:Abbvie: Other: Clinical trial activity (Principal investigator or sub-investigator); Alpine Immune Sciences: Other: Clinical trial activity (Principal investigator or sub-investigator); Annexon Biosciences: Other: Clinical trial activity (Principal investigator or sub-investigator); Astex Pharmaceuticals: Other: Clinical trial activity (Principal investigator or sub-investigator); Baxalta: Other: Clinical trial activity (Principal investigator or sub-investigator); BeiGene: Other: Clinical trial activity (Principal investigator or sub-investigator); Bioverativ: Other: Clinical trial activity (Principal investigator or sub-investigator); CSL Behring: Other: Clinical trial activity (Principal investigator or sub-investigator); Celgene: Other: Clinical trial activity (Principal investigator or sub-investigator); Delta-Fly Pharma: Other: Clinical trial activity (Principal investigator or sub-investigator); Exact Sciences: Other: Clinical trial activity (Principal investigator or sub-investigator); Forma Therapeutics: Other: Clinical trial activity (Principal investigator or sub-investigator); Global Blood Therapeutics: Other: Clinical trial activity (Principal investigator or sub-investigator); Immunovant: Other: Clinical trial activity (Principal investigator or sub-investigator); Incyte: Other: Clinical trial activity (Principal investigator or sub-investigator); Janssen Pharmaceuticals: Other: Clinical trial activity (Principal investigator or sub-investigator); NeoImmuneTech: Other: Clinical trial activity (Principal investigator or sub-investigator); Novartis: Other: Clinical trial activity (Principal investigator or sub-investigator); Novo Nordisk: Other: Clinical trial activity (Principal investigator or sub-investigator); Partner Therapeutics: Other: Clinical trial activity (Principal investigator or sub-investigator); Pharm-Olam: Other: Clinical trial activity (Principal investigator or sub-investigator); Principia Biopharma: Other: Clinical trial activity (Principal investigator or sub-investigator); Salix Pharmaceuticals: Other: Clinical trial activity (Principal investigator or sub-investigator); Sanofi-Aventis: Other: Clinical trial activity (Principal investigator or sub-investigator); Takeda: Other: Clinical trial activity (Principal investigator or sub-investigator); Vifor Pharma: Other: Clinical trial activity (Principal investigator or sub-investigator).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal