Abstract
Developmental hematopoiesis consists of multiple, partially overlapping hematopoietic waves that generate the differentiated blood cells required for embryonic development while establishing a pool of undifferentiated hematopoietic stem cells (HSCs) for postnatal life. This multilayered design in which active hematopoiesis migrates through diverse extra and intraembryonic tissues has made it difficult to define a roadmap for generating HSCs vs non–self-renewing progenitors, especially in humans. Recent single-cell studies have helped in identifying the rare human HSCs at stages when functional assays are unsuitable for distinguishing them from progenitors. This approach has made it possible to track the origin of human HSCs to the unique type of arterial endothelium in the aorta-gonad-mesonephros region and document novel benchmarks for HSC migration and maturation in the conceptus. These studies have delivered new insights into the intricate process of HSC generation and provided tools to inform the in vitro efforts to replicate the physiological developmental journey from pluripotent stem cells via distinct mesodermal and endothelial intermediates to HSCs.
Introduction
Since human pluripotent stem cells (PSCs) were derived from blastocysts1 and subsequently reprogrammed from fibroblasts,2 major efforts have been put into the in vitro generation of blood and immune cells for regenerative or cancer therapies and disease modeling. However, better understanding of human HSC genesis is required for PSC differentiation into transplantable HSCs.3-5 Mammalian developmental hematopoiesis occurs in multiple temporal waves and anatomical niches that generate both differentiated blood cells for the embryo and undifferentiated HSCs for life-long hematopoiesis6-8 (Figure 1). How developing HSCs acquire multilineage differentiation and self-renewal ability remains unanswered. Decoding the intrinsic and extrinsic mechanisms guiding HSCs requires accurate definitions of HSC identity and location, which is challenging because of limited access to human developmental tissues and suboptimal assays to validate immature human HSCs.9 Novel lineage-tracing, barcoding, and in vivo imaging tools have greatly advanced our understanding of mouse developmental hematopoiesis.6,10 However, the discoveries from mice cannot be directly translated to human settings because of the different anatomy of extraembryonic tissues, pregnancy duration and timing of birth, and species-specific regulatory mechanisms11,12 (Figure 1).
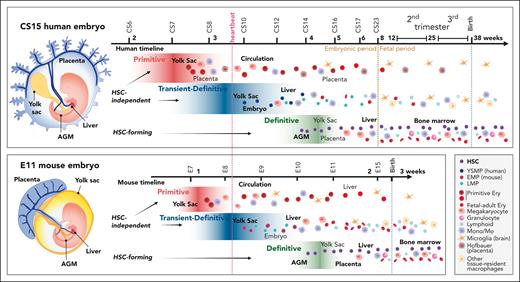
Comparative view of multilayered hematopoiesis during human and mouse development. Hematopoiesis is a conserved developmental process in mammals, but the anatomy of the hematopoietic sites, duration of the hematopoietic waves, and output of the progenitors differ between human and mouse. (Left) Human and mouse embryos are depicted at the stage when HSC emergence peaks (CS15; 5 weeks; E11). Although embryonic structures and the main vascular circuitry are similar, the anatomy of extraembryonic tissues is different. The human placenta is villous type and surrounds the embryo and amniotic membrane, whereas the yolk sac is a balloon-like appendage inside the amnion. The mouse placenta is labyrinthine-type, and the yolk sac surrounds the embryo and amnion. In both species, blood flows from the aorta through the vitelline and umbilical arteries to the yolk sac and the placenta and returns to the embryo through the liver via vitelline and umbilical veins. Human hematopoiesis starts by 2.5 weeks (CS7) in the yolk sac, with the first, primitive progenitor wave, during which the main products are nucleated primitive erythroblasts that enter circulation. In addition, the precirculation placenta generates macrophages (Hofbauer cells) that assist in primitive erythroblast enucleation in the placental villi. At 3.25 weeks (CS8-CS9) the second, transient- (or pro) definitive wave of human hematopoiesis is initiated in the yolk sac and possibly in the embryo proper as well. In human, the second wave starts with YSMPs, followed by LMPs of yet unknown origin. HSC-independent progenitors give rise to tissue-resident macrophages, such as microglia, Langerhans cells, and Kupffer cells that can last into adulthood and HSC-independent lymphoid populations. HSC-independent macrophages may have diverse origins, and most brain microglia generation in mice is linked to primitive rather than second wave progenitors. Many of second wave progenitors colonize the liver where they differentiate to blood and immune cells to support development. Between 4 and 6 weeks (CS14-CS16) a third, HSC-forming, definitive hematopoietic wave arises in the AGM region and produces nascent HSCs. HSCs first migrate through the placenta and yolk sac before they seed the liver (CS17). During these transitions HSCs undergo maturation, limited expansion, and some give rise to terminally differentiated progeny. HSCs start generating multilineage progeny already in the first trimester liver and move to the BM during the second trimester to sustain postnatal hematopoiesis. Mouse hematopoiesis is aligned according to comparable developmental stages as in human. Because mouse embryogenesis is compressed into a much shorter timeframe, a greater overlap of developmental events and hematopoietic populations is apparent. The end of mouse gestation (3 weeks) compares to the early fetal period (9 weeks) of human development. Some progenitor populations differ in their lineage output, such as mouse yolk sac transient definitive progenitors are highly primed for erythromyeloid differentiation (EMPs), whereas the corresponding yolk sac progenitors in human show myeloid skewing (YSMPs), and the first signs of liver erythropoiesis link to HSCs. Weeks are referred to as developmental age, (ie, weeks after fertilization, which is 2 weeks less than gestational weeks or weeks from the last menstrual cycle). Dotted lines depict developmental milestones, such as the onset of heartbeat, transition from embryonic to fetal period during human development, and birth, which occurs at very different developmental stages in mouse and human. The main hematopoietic cell types are described (bottom right). EMPs, erythromyeloid progenitors; Ery, erythroid cell; Mono/Mφ, monocyte/macrophage.
Comparative view of multilayered hematopoiesis during human and mouse development. Hematopoiesis is a conserved developmental process in mammals, but the anatomy of the hematopoietic sites, duration of the hematopoietic waves, and output of the progenitors differ between human and mouse. (Left) Human and mouse embryos are depicted at the stage when HSC emergence peaks (CS15; 5 weeks; E11). Although embryonic structures and the main vascular circuitry are similar, the anatomy of extraembryonic tissues is different. The human placenta is villous type and surrounds the embryo and amniotic membrane, whereas the yolk sac is a balloon-like appendage inside the amnion. The mouse placenta is labyrinthine-type, and the yolk sac surrounds the embryo and amnion. In both species, blood flows from the aorta through the vitelline and umbilical arteries to the yolk sac and the placenta and returns to the embryo through the liver via vitelline and umbilical veins. Human hematopoiesis starts by 2.5 weeks (CS7) in the yolk sac, with the first, primitive progenitor wave, during which the main products are nucleated primitive erythroblasts that enter circulation. In addition, the precirculation placenta generates macrophages (Hofbauer cells) that assist in primitive erythroblast enucleation in the placental villi. At 3.25 weeks (CS8-CS9) the second, transient- (or pro) definitive wave of human hematopoiesis is initiated in the yolk sac and possibly in the embryo proper as well. In human, the second wave starts with YSMPs, followed by LMPs of yet unknown origin. HSC-independent progenitors give rise to tissue-resident macrophages, such as microglia, Langerhans cells, and Kupffer cells that can last into adulthood and HSC-independent lymphoid populations. HSC-independent macrophages may have diverse origins, and most brain microglia generation in mice is linked to primitive rather than second wave progenitors. Many of second wave progenitors colonize the liver where they differentiate to blood and immune cells to support development. Between 4 and 6 weeks (CS14-CS16) a third, HSC-forming, definitive hematopoietic wave arises in the AGM region and produces nascent HSCs. HSCs first migrate through the placenta and yolk sac before they seed the liver (CS17). During these transitions HSCs undergo maturation, limited expansion, and some give rise to terminally differentiated progeny. HSCs start generating multilineage progeny already in the first trimester liver and move to the BM during the second trimester to sustain postnatal hematopoiesis. Mouse hematopoiesis is aligned according to comparable developmental stages as in human. Because mouse embryogenesis is compressed into a much shorter timeframe, a greater overlap of developmental events and hematopoietic populations is apparent. The end of mouse gestation (3 weeks) compares to the early fetal period (9 weeks) of human development. Some progenitor populations differ in their lineage output, such as mouse yolk sac transient definitive progenitors are highly primed for erythromyeloid differentiation (EMPs), whereas the corresponding yolk sac progenitors in human show myeloid skewing (YSMPs), and the first signs of liver erythropoiesis link to HSCs. Weeks are referred to as developmental age, (ie, weeks after fertilization, which is 2 weeks less than gestational weeks or weeks from the last menstrual cycle). Dotted lines depict developmental milestones, such as the onset of heartbeat, transition from embryonic to fetal period during human development, and birth, which occurs at very different developmental stages in mouse and human. The main hematopoietic cell types are described (bottom right). EMPs, erythromyeloid progenitors; Ery, erythroid cell; Mono/Mφ, monocyte/macrophage.
Developmental hematopoiesis was classically divided into 2 waves. The primitive hematopoietic wave, mainly derived from the extraembryonic yolk sac, generates the first blood cells in the precirculation conceptus and provides the embryo with oxygen, immune protection, and tissue-remodeling capabilities. The definitive wave, traditionally linked to the embryo, generates self-renewing HSCs that make differentiated blood cells for the lifetime. However, recent studies have shown that developmental hematopoiesis cannot be explained by only 2 waves and locations, given that several intermediate progenitors (often termed transient definitive or prodefinitive6,7) with properties between primitive hematopoietic cells and HSCs were discovered in both extraembryonic and intraembryonic tissues. These concepts have been summarized in several comprehensive reviews.4,6,8,13,14 Because of the inconsistencies in the literature for the term “definitive” hematopoiesis (ie, anything not primitive or only HSC-generating), we refer to the true definitive hematopoietic wave as the “HSC-forming” wave. Because developing HSCs are intermixed with circulating HSC-independent progenitors, pinpointing their unique regulatory programs has been challenging.4,7 Single-cell technologies have provided new tools to identify human HSCs based on their molecular signatures (Table 115-31). In this review, we summarize how these studies have shaped our understanding of the genesis of human HSCs and identify areas for future studies to facilitate robust HSC generation in vitro.
Human HSC ontogeny and characteristics
| Human hematopoietic cell type . | Description and functional properties . | RNA expression signature . | Protein markers . | References . |
|---|---|---|---|---|
| HSC endothelial precursors | ||||
| AGM AE CS13/15 wk 4 to wk 5 | Arterial ECs from the AGM region lining the aorta | RUNX1–HOXA9+MEIS2+SOX17+ | CDH5+CD34+CD90+CXCR4+GJA5+ CD43–CD45– | 15,16 |
| AGM pre-HE CS13/15 wk 4 to wk 5 | Subset of AE cells from the aorta preceding the activation of hematopoietic transition | RUNX1–HOXA9+MEIS2+SOX17+IL-33+ ALDH1A1+DKK1+ | CDH5+CD34+CD90+CXCR4+GJA5+IL-33+ALDH1A1+CD43–CD45– | 15 |
| AGM HE CS13/15 wk 4 to wk 5 | Subset of AE on the ventral side of dorsal aorta undergoing EHT (RUNX1 expression without SPN/PTPRC activation) | RUNX1+HOXA9+MEIS2+SOX17+ALDH1A1+ DKK1+ | CDH5+CD34+CD90+CD44+ KCNK17+CD43–CD45– | 15,16 |
| HSC | ||||
| AGM HSCs CS14/15 wk 4 to wk 5 | HSC emerging via IAHC, undifferentiated state with immature endothelial/megakaryocytic surface features and displaying functional immaturity. Rare highly potent transplantable HSCs. | RUNX1+MLLT3+HOXA9+SPINK2+HLF+MECOM+LIN28B+IGFBP2+HOXB9+ | CD34+CD90+KCNK17+CD43+CD45+ CDH5+EMCN+ACE+PROCR+ | 15,17,18 |
| Yolk sac HSCs CS14/CS16 | HSCs displaying functional immaturity but diminished endothelial features compared with AGM HSCs. Transplantable HSCs found at CS16. Erythroid priming and transcriptional similarity to CS17 liver HSCs observed. | RUNX1+MLLT3+HOXA9+SPINK2+HLF+MECOM+LIN28B+IGFBP2+HOXB9+ | CD34+CD90+CD43+CD45+ CDH5+/−ACE+PROCR+ | 15,17,19 |
| Placental HSCs CS14 to wk 9 | HSCs displaying functional immaturity but diminished endothelial features compared with AGM HSCs. Erythroid priming and transcriptional similarity to CS17 liver HSCs are observed. Presence of GPI80+ HSPCs observed at 5 wk. | RUNX1+MLLT3+HOXA9+SPINK2+HLF+MECOM+LIN28B+IGFBP2+HOXB9+ | CD34+CD90+CD43+CD45+GPI80+ CDH5+/−ACE+PROCR+ | 15,19,20 |
| Embryonic liver HSC CS17/20 wk 6 to wk 7 | HSC displaying functional immaturity but showing suppression of endothelial features. Erythro-megakaryocyte-myeloid differentiation-primed. | RUNX1+MLLT3+HOXA9+SPINK2+HLF+MECOM+IGFBP2+LIN28B+HOXB9+ | CD34+CD90+CD43+CD45+GPI80+ ACE+PROCR+ | 15,17,19 |
| First trimester fetal liver HSC from wk 8 to wk 12 | Transplantable HSCs, progressive decline of fetal programs, gradual acquisition of multipotency and quiescence, and increased expression of MHC class I and II and PROM1 | RUNX1+MLLT3+HOXA9+SPINK2+HLF+MECOM+HEMGN | CD34+CD90+CD43+CD45+GPI80+ACE+PROCR+PROM1+HLA-DR+ | 15,19,21 |
| Second trimester liver HSCs from wk 13 to wk 20 | Transplantable HSCs, gradual loss of fetal properties and transition to quiescence, and robust expression of MHC class I and II and PROM1 | RUNX1+MLLT3+HOXA9+SPINK2+HLF+MECOM+HEMGN+MSI2hi+ | CD34+CD90+CD43+CD45+GPI80+ACE+PROCR+PROM1+HLA-DR+ | 15,21-26 |
| Second trimester fetal BM HSCs | Transplantable HSCs, further transition to quiescence, and robust expression of MHC class I and II and PROM1 | RUNX1+MLLT3+HOXA9+SPINK2+HLF+MECOM+HEMGN+MSI2hi | CD34+CD90+CD43+CD45+GPI80+ACE+PROCR+PROM1+HLA-DR+ | 20,23,25-27 |
| Cord blood HSCs | Transplantable HSCs, complete acquisition of functional maturation signature, and robust expression of MHC class I and II | RUNX1+MLLT3+HOXA9+SPINK2+ HLF+MECOM+HEMGN+MSI2hi | CD34+CD90+CD43+CD45+GPI80–ACE+ PROCR+PROM1+HLA-DR+SELL+EMCN+ CD164+ | 15,28-31 |
| Human hematopoietic cell type . | Description and functional properties . | RNA expression signature . | Protein markers . | References . |
|---|---|---|---|---|
| HSC endothelial precursors | ||||
| AGM AE CS13/15 wk 4 to wk 5 | Arterial ECs from the AGM region lining the aorta | RUNX1–HOXA9+MEIS2+SOX17+ | CDH5+CD34+CD90+CXCR4+GJA5+ CD43–CD45– | 15,16 |
| AGM pre-HE CS13/15 wk 4 to wk 5 | Subset of AE cells from the aorta preceding the activation of hematopoietic transition | RUNX1–HOXA9+MEIS2+SOX17+IL-33+ ALDH1A1+DKK1+ | CDH5+CD34+CD90+CXCR4+GJA5+IL-33+ALDH1A1+CD43–CD45– | 15 |
| AGM HE CS13/15 wk 4 to wk 5 | Subset of AE on the ventral side of dorsal aorta undergoing EHT (RUNX1 expression without SPN/PTPRC activation) | RUNX1+HOXA9+MEIS2+SOX17+ALDH1A1+ DKK1+ | CDH5+CD34+CD90+CD44+ KCNK17+CD43–CD45– | 15,16 |
| HSC | ||||
| AGM HSCs CS14/15 wk 4 to wk 5 | HSC emerging via IAHC, undifferentiated state with immature endothelial/megakaryocytic surface features and displaying functional immaturity. Rare highly potent transplantable HSCs. | RUNX1+MLLT3+HOXA9+SPINK2+HLF+MECOM+LIN28B+IGFBP2+HOXB9+ | CD34+CD90+KCNK17+CD43+CD45+ CDH5+EMCN+ACE+PROCR+ | 15,17,18 |
| Yolk sac HSCs CS14/CS16 | HSCs displaying functional immaturity but diminished endothelial features compared with AGM HSCs. Transplantable HSCs found at CS16. Erythroid priming and transcriptional similarity to CS17 liver HSCs observed. | RUNX1+MLLT3+HOXA9+SPINK2+HLF+MECOM+LIN28B+IGFBP2+HOXB9+ | CD34+CD90+CD43+CD45+ CDH5+/−ACE+PROCR+ | 15,17,19 |
| Placental HSCs CS14 to wk 9 | HSCs displaying functional immaturity but diminished endothelial features compared with AGM HSCs. Erythroid priming and transcriptional similarity to CS17 liver HSCs are observed. Presence of GPI80+ HSPCs observed at 5 wk. | RUNX1+MLLT3+HOXA9+SPINK2+HLF+MECOM+LIN28B+IGFBP2+HOXB9+ | CD34+CD90+CD43+CD45+GPI80+ CDH5+/−ACE+PROCR+ | 15,19,20 |
| Embryonic liver HSC CS17/20 wk 6 to wk 7 | HSC displaying functional immaturity but showing suppression of endothelial features. Erythro-megakaryocyte-myeloid differentiation-primed. | RUNX1+MLLT3+HOXA9+SPINK2+HLF+MECOM+IGFBP2+LIN28B+HOXB9+ | CD34+CD90+CD43+CD45+GPI80+ ACE+PROCR+ | 15,17,19 |
| First trimester fetal liver HSC from wk 8 to wk 12 | Transplantable HSCs, progressive decline of fetal programs, gradual acquisition of multipotency and quiescence, and increased expression of MHC class I and II and PROM1 | RUNX1+MLLT3+HOXA9+SPINK2+HLF+MECOM+HEMGN | CD34+CD90+CD43+CD45+GPI80+ACE+PROCR+PROM1+HLA-DR+ | 15,19,21 |
| Second trimester liver HSCs from wk 13 to wk 20 | Transplantable HSCs, gradual loss of fetal properties and transition to quiescence, and robust expression of MHC class I and II and PROM1 | RUNX1+MLLT3+HOXA9+SPINK2+HLF+MECOM+HEMGN+MSI2hi+ | CD34+CD90+CD43+CD45+GPI80+ACE+PROCR+PROM1+HLA-DR+ | 15,21-26 |
| Second trimester fetal BM HSCs | Transplantable HSCs, further transition to quiescence, and robust expression of MHC class I and II and PROM1 | RUNX1+MLLT3+HOXA9+SPINK2+HLF+MECOM+HEMGN+MSI2hi | CD34+CD90+CD43+CD45+GPI80+ACE+PROCR+PROM1+HLA-DR+ | 20,23,25-27 |
| Cord blood HSCs | Transplantable HSCs, complete acquisition of functional maturation signature, and robust expression of MHC class I and II | RUNX1+MLLT3+HOXA9+SPINK2+ HLF+MECOM+HEMGN+MSI2hi | CD34+CD90+CD43+CD45+GPI80–ACE+ PROCR+PROM1+HLA-DR+SELL+EMCN+ CD164+ | 15,28-31 |
Characteristics of different human HSC endothelial precursors and HSC maturation stages.
The paradox of developmental hematopoiesis: blood formation before HSCs
Primitive hematopoiesis in extraembryonic tissues
The first blood-forming cells in mammalian embryos arise not from HSCs but from primitive hematopoietic progenitors.32,33 These differentiation-primed progenitors originate from the precirculation yolk sac (YS) at Carnegie stages 7-8 (CS7-8) of human development34 (16-18.5 days after fertilization; embryonic day 7-8 [E7-8] in mice) and generate primitive erythroblasts, megakaryocytes, and macrophages (Figure 1). Primitive hematopoiesis was first observed in early morphological studies of human embryos35 and has recently been validated via single-cell RNA-sequencing (scRNA-seq) in CS7 conceptus.36 Primitive erythroblasts are initially nucleated, larger than definitive erythroid cells, and express embryonic ζ/ε globins with higher oxygen carrying capacity.37,38 They enter circulation when the heartbeat begins (21-23 days, CS1039,40). Upon reaching placental vasculature, primitive erythroblasts enucleate between 5 and 10 weeks by interacting with macrophages (Hofbauer cells) in placental villous stroma,41 after which they are replaced by a new wave of enucleated fetal erythroid cells expressing α/γ globins, which differentiate in the liver. Human placental macrophages are generated in situ without input from circulating cells,41,42 implying that multiple sources of precirculation macrophages contribute to human primitive hematopoiesis (Figure 1). The origin of macrophages in mouse placenta remains controversial.43-45
Transient definitive hematopoietic progenitor waves
Primitive hematopoiesis is followed by a second wave of HSC-independent progenitors (transient definitive or prodefinitive) that have partial multilineage differentiation capacity but do not differentiate at their place of origin33,46 (Figure 1). In mice, the second wave is dominated by yolk sac–derived CD41+cKit+CD16/32+ erythromyeloid progenitors that jump-start fetal liver (FL) erythropoiesis at approximately E10.5.47 Recent studies also revealed lymphoid potential among HSC-independent progenitors, overturning the dogma that lymphoid potential only associates with HSCs.48-52
Lineage-tracing studies also linked yolk sac HSC–independent hematopoietic progenitors to long-lived progeny, including brain microglia and other tissue-resident macrophages,53-55 and unique lymphoid populations56 that persist through adulthood and participate in neurogenerative diseases57 and neoplasia,58,59 respectively (Figure 1). These populations may have several cellular origins in the yolk sac. In mice, most tissue-resident macrophages arise from Kitl-dependent second wave progenitors, whereas brain microglia originate from Kitl-independent progenitors.60
The second hematopoietic wave has been less well-defined in human embryos. scRNA-seq analysis helped identify early human yolk sac–derived myeloid progenitors (YSMPs) that seed the liver by CS12,61 likely representing human transient definitive hematopoiesis. Comparison of CS11 YSMPs with nascent HSCs (identified by the presence of HSC signature RUNX1+HOXA9+MLLT3+MECOM+HLF+SPINK2+)15 using scRNA-seq showed that although YSMPs express many genes associated with HSCs (HLF, RUNX1, and SPINK2), they lack HSC hallmarks (medial HOXA genes and robust MLLT3 and MECOM expression28,62,63) and express embryonic signature genes (LIN28A).15 scRNA-seq analysis of CS10 embryo also helped detect intraembryonic hematopoietic progenitors that are similar to CS11 YSMPs and linked to the embryonic hemogenic endothelium (HE), which is distinct from HSC-forming HE in CS13 to CS15 aorta-gonad-mesonephros (AGM).15,16 The mouse embryo can generate HSC-independent lympho-myeloid progenitors (LMPs) and multipotent progenitors (MPPs) that partially overlap with HSC generation,49,52,64-66 but it is unclear whether there is a mouse counterpart for human CS10 HE observed at the onset of the heartbeat. The compressed developmental timeline in mice67 likely leads to greater overlap between progenitor- and HSC-forming hemogenic waves (Figure 1).
B- and T-lymphoid potential, previously considered a proxy for the HSC lineage in in vitro assays, has been linked to HSC-independent progenitors in both mouse and human embryos.16,32,52,61,68 scRNA-seq of CS14 human conceptus identified a SPINK2+–interleukin–7 receptor–positive (SPINK2+IL-7+) LMPs that are distinct from early CS10 to CS11 myeloid progenitors and from CS13 to CS15 HSCs. At CS14, they are found mainly in the liver, head, and heart, suggesting that they enter circulation and colonize the liver before HSCs. The CS14 liver SPINK2+ progenitors lack the expression of HSC signature genes that regulate HSC fate (HLF, HOXA9, MLLT3, and MECOM) (Table 215,16,62,63,69-96) and associate with lymphoid (IL-7R; EBF1) and macrophage (C1QA, MRC, and CX3CR1) trajectories in CS14-17 livers.15 Similar lymphoid-biased IL-7R+CD7hi progenitor was reported in CS15 liver in another independent study.61 Between weeks 7 and 9, several lymphoid populations appear in the liver, including thymus-seeding progenitors and progenitors for B-cells and innate lymphoid cells.61,97,98 Further investigations are needed to discriminate between HSC-dependent or HSC-independent origin for the various immune progenitors. Nevertheless, differentiation trajectories suggest that HSC-derived lymphopoiesis begins in the liver by the end of embryonic period (8 weeks), as HSC-independent SPINK2+IL-7R+ LMPs disappear.15
Transcriptional regulation of human HSC specification and maintenance
| Human HSC transcriptional regulators . | Role in hematopoiesis . | Functional validation . | References . |
|---|---|---|---|
| HSC specification | |||
| SCL/TAL1 | Promotes HE establishment and suppresses cardiac fate during mesoderm specification. Augments hematopoietic specification in hPSC cultures. | Mouse KO, mESC KO differentiation, and hESC OE model | 69,70-72 |
| SOX17 | Essential for establishment of arterial identity, definitive HE specification, and EHT. Marks definitive HE during hPSC differentiation. | Mouse KO, mESC KO differentiation, hESC reporters, and loss of function/OE models. | 63,73-76 |
| Medial HOXA genes | HOXA5, 7, and 9 are landmarks of specification to definitive hematopoietic fate in vitro and in vivo, HOXA9 associates with HSC proliferation and HOXA7 with suppressing primitive hematopoiesis associated programs in human fetal HSCs | Mouse KO and human FL-HSC KD/OE | 15,62,63,74,77-80 |
| RUNX1 | Necessary for IAHC formation in both HSC-independent and HSC-forming EHT. Indicates HE specification during human development and in hPSC cultures. | Mouse KO, mESC differentiation, and hESC reporters | 15,63,73,81 |
| MYB | Required during definitive hematopoiesis for HSC self-renewal and suppression of differentiation programs | Mouse KO and hESC reporters | 16,82,83 |
| GFI1/GFI1B | Promote hematopoietic identity by suppressing endothelial program and inducing quiescence | Mouse KO and small molecule inhibitors during hESC differentiation | 84-87 |
| HSC maintenance | |||
| MLLT3 | Essential for human HSC self-renewal. Sustains HSC transcriptional network via DOT1L. Expressed from pre-HE stage onwards. Levels increase during human HSC maturation. Expands functional HSCs. | KD and OE in human FL HSCs and CB-HSCs | 15,88 |
| HLF | Defines undifferentiated HSPC and promotes HSC quiescence. Highly specific for undifferentiated HSPC but expressed also in some second wave progenitors in human, although not observed in mouse erythromyeloid progenitors. Essential for human HSC function. | Mouse KOs, mouse reporters, and human CB HSC reporters. KD in human FL HSCs | 15,88-92 |
| MECOM | Maintains HSC proliferation in a dosage-dependent manner. Expressed highly in EC throughout EHT and HSCs. High expression levels define LT-HSC lineage. Essential for human HSC function. | Mouse KOs, mouse reporters. KD in human FL HSCs | 15,88,91,93,94 |
| MSI2 | Promotes human HSC expansion through AHR signaling inhibition | Mouse KO, human HSC KD/OE | 95,96 |
| Human HSC transcriptional regulators . | Role in hematopoiesis . | Functional validation . | References . |
|---|---|---|---|
| HSC specification | |||
| SCL/TAL1 | Promotes HE establishment and suppresses cardiac fate during mesoderm specification. Augments hematopoietic specification in hPSC cultures. | Mouse KO, mESC KO differentiation, and hESC OE model | 69,70-72 |
| SOX17 | Essential for establishment of arterial identity, definitive HE specification, and EHT. Marks definitive HE during hPSC differentiation. | Mouse KO, mESC KO differentiation, hESC reporters, and loss of function/OE models. | 63,73-76 |
| Medial HOXA genes | HOXA5, 7, and 9 are landmarks of specification to definitive hematopoietic fate in vitro and in vivo, HOXA9 associates with HSC proliferation and HOXA7 with suppressing primitive hematopoiesis associated programs in human fetal HSCs | Mouse KO and human FL-HSC KD/OE | 15,62,63,74,77-80 |
| RUNX1 | Necessary for IAHC formation in both HSC-independent and HSC-forming EHT. Indicates HE specification during human development and in hPSC cultures. | Mouse KO, mESC differentiation, and hESC reporters | 15,63,73,81 |
| MYB | Required during definitive hematopoiesis for HSC self-renewal and suppression of differentiation programs | Mouse KO and hESC reporters | 16,82,83 |
| GFI1/GFI1B | Promote hematopoietic identity by suppressing endothelial program and inducing quiescence | Mouse KO and small molecule inhibitors during hESC differentiation | 84-87 |
| HSC maintenance | |||
| MLLT3 | Essential for human HSC self-renewal. Sustains HSC transcriptional network via DOT1L. Expressed from pre-HE stage onwards. Levels increase during human HSC maturation. Expands functional HSCs. | KD and OE in human FL HSCs and CB-HSCs | 15,88 |
| HLF | Defines undifferentiated HSPC and promotes HSC quiescence. Highly specific for undifferentiated HSPC but expressed also in some second wave progenitors in human, although not observed in mouse erythromyeloid progenitors. Essential for human HSC function. | Mouse KOs, mouse reporters, and human CB HSC reporters. KD in human FL HSCs | 15,88-92 |
| MECOM | Maintains HSC proliferation in a dosage-dependent manner. Expressed highly in EC throughout EHT and HSCs. High expression levels define LT-HSC lineage. Essential for human HSC function. | Mouse KOs, mouse reporters. KD in human FL HSCs | 15,88,91,93,94 |
| MSI2 | Promotes human HSC expansion through AHR signaling inhibition | Mouse KO, human HSC KD/OE | 95,96 |
Key transcription factors functionally implicated in human HSC development in vivo or in vitro and their major roles are indicated.
AHR, aryl hydrocarbon receptor; CB, cord blood; ESC, embryonic stem cells; h, human; KD, knockdown; KO, knockout; m, murine; OE, overexpression.
scRNA-seq of other human developmental tissues has revealed unexpected locations and differentiation trajectories of hematopoiesis, including erythropoiesis in the skin and kidney during first trimester and hematopoietic stem and progenitor cell (HSPC) formation in the lung during second trimester.16,22,23,99 Future studies will elucidate the origin and role of these hematopoietic populations.
Developmental journey of HSCs
Generation of immature HSCs in the AGM region
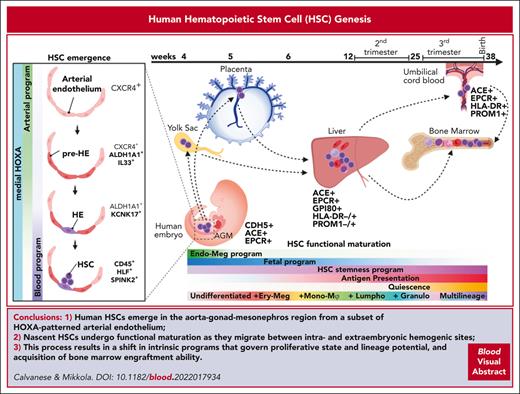
The third, definitive hematopoiesis wave generates HSCs, but their anatomical and cellular origin has been difficult to pinpoint. The AGM is a functionally validated primary site of HSC emergence in mice and humans, whereas the contribution of extraembryonic tissues is still under investigation. Human HSCs emerge at CS14 to CS16 (4-5 weeks) on the ventral side of the dorsal aorta via intra-aortic hematopoietic clusters (IAHCs)40 (Figures 2 and 315; Table 1), which were first observed in early histological studies.100 Hematopoietic potential of CD34+ cells from CS13 to CS14 AGM vasculature and IAHCs was established using long-term culture–initiating cell stroma coculture,40 but there was no proof whether they represent HSCs or progenitors. HSC quantification using the gold-standard human HSC functional assay and transplantation to immunodeficient mice showed rare transplantable HSCs in human AGM (1 per embryo),17 similar to that in mice,101,102 suggesting that most cells in IAHCs are HSC-independent progenitors or immature HSCs that cannot repopulate adult bone marrow (BM). Nonetheless, the presence of rare cells with extensive regenerative potential in immunodeficient mice confirmed the intrinsic potency of AGM HSCs.17,18 Single-cell studies in mouse embryos identified a molecularly distinct HSC population that is much larger than those detected using transplantation assays, supporting the hypothesis that AGM HSCs are functionally immature.103 This conclusion was validated by lineage-tracing and barcoding studies10 and optimized in vitro maturation cultures that derived transplantable mouse HSCs from immature pre-HSCs and endothelial precursors.66,98,104,105
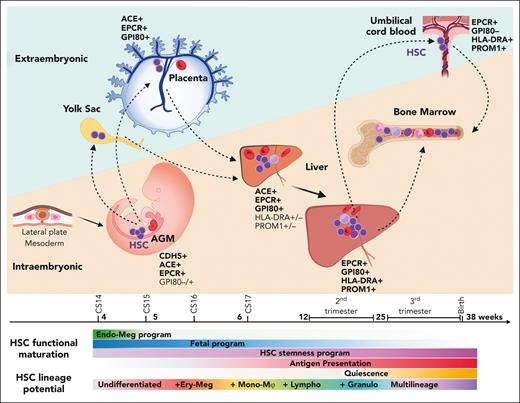
The developmental journey of human HSCs. Schematic diagram showing human HSCs throughout gestation as they migrate between intra and extraembryonic niches. HSCs are generated in the lateral plate mesoderm–derived AGM region, where they show an undifferentiated and developmentally immature phenotype. As HSCs emerge from the IAHCs, they follow circulation to the placenta and the yolk through the main arterial outlets of the aorta, the umbilical and vitelline arteries. In extraembryonic sites, HSCs suppress endothelial identity and show transcriptional priming for erythro-megakaryocytic fates. HSCs return to the embryo after CS17 as they colonize the liver for developmental maturation and initiate lineage differentiation. Over time, HSCs acquire multilineage differentiation ability as they switch from erythro-megakaryocytic bias to multilineage potential, including myeloid and lymphoid lineages. They express fetal HSC surface marker GPI80 and acquire HSC maturity markers (PROM1/CD133 and antigen presentation machinery). They gradually lose intrinsic fetal properties and boost HSC self-renewal program, while transitioning toward homeostatic state. During the second trimester, HSCs transition to the BM, where they transition to deeper quiescence and complete developmental maturation. HSC shuttling between intra- and extraembryonic sites (dashed arrows) continues throughout gestation as evidenced by their presence in the placental umbilical cord blood at birth.
The developmental journey of human HSCs. Schematic diagram showing human HSCs throughout gestation as they migrate between intra and extraembryonic niches. HSCs are generated in the lateral plate mesoderm–derived AGM region, where they show an undifferentiated and developmentally immature phenotype. As HSCs emerge from the IAHCs, they follow circulation to the placenta and the yolk through the main arterial outlets of the aorta, the umbilical and vitelline arteries. In extraembryonic sites, HSCs suppress endothelial identity and show transcriptional priming for erythro-megakaryocytic fates. HSCs return to the embryo after CS17 as they colonize the liver for developmental maturation and initiate lineage differentiation. Over time, HSCs acquire multilineage differentiation ability as they switch from erythro-megakaryocytic bias to multilineage potential, including myeloid and lymphoid lineages. They express fetal HSC surface marker GPI80 and acquire HSC maturity markers (PROM1/CD133 and antigen presentation machinery). They gradually lose intrinsic fetal properties and boost HSC self-renewal program, while transitioning toward homeostatic state. During the second trimester, HSCs transition to the BM, where they transition to deeper quiescence and complete developmental maturation. HSC shuttling between intra- and extraembryonic sites (dashed arrows) continues throughout gestation as evidenced by their presence in the placental umbilical cord blood at birth.
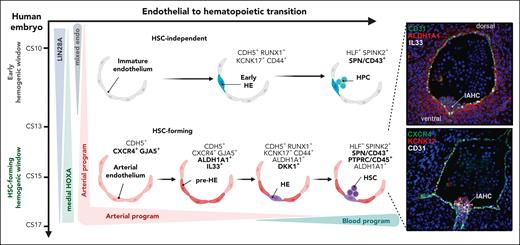
Specification and emergence of HSCs via EHT in the human AGM region. Schematic representation of the establishment of HSC-forming HE in the human AGM region. At CS14/15, an arterially specified HOXA-patterned EC gives rise to an arterial subset termed pre-HE, characterized by ALDH1A1 (RA-signaling) and IL-33 expression. HE is specified from pre-HE by the hematopoietic transcription factor RUNX1 in cells that upregulate KCNK17, CD44, and WNT inhibitors (DKK1), and gives rise to SPINK2+CD45+ nascent HSCs. In the earlier embryo (CS10-11), HE that generates HSC-independent progenitors is specified from immature endothelial precursors that express embryonic genes (LIN28A) and lack HOXA patterning and arterial identity. (Right) Immunofluorescence validation of stage-specific markers indicate the emergence of human HSCs from IAHC in sections of CS15 human AGM. Modified from Calvanese et al.15
Specification and emergence of HSCs via EHT in the human AGM region. Schematic representation of the establishment of HSC-forming HE in the human AGM region. At CS14/15, an arterially specified HOXA-patterned EC gives rise to an arterial subset termed pre-HE, characterized by ALDH1A1 (RA-signaling) and IL-33 expression. HE is specified from pre-HE by the hematopoietic transcription factor RUNX1 in cells that upregulate KCNK17, CD44, and WNT inhibitors (DKK1), and gives rise to SPINK2+CD45+ nascent HSCs. In the earlier embryo (CS10-11), HE that generates HSC-independent progenitors is specified from immature endothelial precursors that express embryonic genes (LIN28A) and lack HOXA patterning and arterial identity. (Right) Immunofluorescence validation of stage-specific markers indicate the emergence of human HSCs from IAHC in sections of CS15 human AGM. Modified from Calvanese et al.15
To track the emergence and maturation of HSCs in human embryos in which in vivo interrogations are not possible and functional maturation in vitro has not been achieved,18 scRNA-seq analysis was used to distinguish human HSCs from their differentiated progeny and HSC-independent progenitors throughout ontogeny. This distinction could be done using a 6 gene HSC signature that consists of transcription factors governing definitive HE or HSC specification (RUNX1 and HOXA9) or maintenance (MLLT3, MECOM, and HLF) (Table 2), and HSC or progenitor gene SPINK2.15 CS14-15 AGM harbored a molecularly uniform population of hundreds of nascent HSCs, supporting the concept that human AGM HSCs are more numerous than those detected using transplantation assay and functionally more immature.
HSC maturation in the fetal liver
The 6-gene HSC signature helped track the transition of HSCs between the AGM and liver approximately at CS17 (6 developmental weeks), after liver hematopoiesis was already established (Figure 2; Table 1). The liver is the main site for active hematopoiesis throughout the first and second trimesters and a “melting pot” for the different hematopoietic waves. The liver rudiment emerges at approximately CS10 (week 3) and becomes populated first by primitive erythroid cells, macrophages, and circulating progenitors. Immunostaining and in vitro studies suggested that the CS12 liver is seeded by CD34+CD45+ hematopoietic progenitors,40 which single-cell studies identified as myeloid-primed YSMPs61 (Figure 1). An early landmark study uncovered multiple lineages of hematopoietic cells in the liver at CS13/CS14.106 These likely represent circulating primitive erythroid cells and myeloid or LMPs and their progeny, because the liver is colonized by molecularly defined HSCs only at 6 weeks15 and transplantable HSCs only after weeks 7 or 817,18,21,107 (Table 1). These observations imply that human liver hematopoiesis, like that in mice, is sequentially initiated by HSC-independent progenitors, followed by immature HSCs.
Previous studies identified surface markers to isolate human embryonic (<8 weeks) and fetal HSCs, including angiotensin I converting enzyme (ACE) (from AGM to CB),19,28,107 GPI-80 (VNN2, from placenta to fetal BM),20,24 and EPCR (PROCR, all stages including cultured CB).24,108,109 However, little has been known about the process how nascent human HSCs acquire functional maturity and robust BM engraftment ability. scRNA-seq analysis of HLF+ human HSCs throughout development uncovered gene expression changes, reflecting HSC maturation15 (Figure 2). Although HSC identity is evident upon HSC emergence based on the expression of known HSC transcription factors, their maturation to transplantable HSCs involves temporal changes in transcriptional programs, reflecting unique surface phenotypes, biological processes, and behaviors. Moreover, their differentiation trajectories evolve from predominantly erythro-megakaryocytic during the first trimester to myelo-lymphoid and multilineage output in the second trimester.15,22 Concomitantly, they suppress endothelial and megakaryocytic surface features and embryonic or early fetal intrinsic programs (LIN28B, IGFBP2, and posterior HOXB genes) and proliferation genes, increase the expression of HSC stemness regulators (MLLT3, HLF, and MSI2), and acquire maturity markers PROM1/CD133 and major histocompatibility complex (MHC) class II molecules (Figure 2; Table 1). Mouse studies revealed that HSCs also functionally require the expression of MHC class I and II genes.110,111 Together, the fetal HSC identity marker and newly identified maturation surface markers facilitate evaluating the human fetal HSC maturity stage via flow cytometry15 (Table 1; Figure 2).
The dogma that fetal liver is the site for HSC expansion has been recently questioned using mice. Previous studies evaluated HSC expansion based on the increase in engraftable HSCs during development.112 However, confetti-based lineage tracing implied that HSCs with life-long potential do not expand massively in the liver, and the immunophenotypic HSCs that proliferate robustly between E12.5 and E14.5 are prone to differentiation rather than symmetric self-renewal.113 This suggests that the increase in transplantable HSCs in mouse liver is largely explained by functional maturation rather than extensive HSC expansion. In humans, scRNA-seq analysis of HLF+ fetal liver HSCs over developmental time evidenced declining expression of proliferation genes at the end of the embryonic period, implying a shift toward homeostatic HSC state already during the first trimester.15
HSCs in extraembryonic tissues preceding liver colonization
In mice, HSCs were found in extraembryonic yolk sac, placenta, and vitelline and umbilical arteries, and hematopoiesis was also reported in the head and the heart.41,114-121 The contribution of these sites to human developmental hematopoiesis remains unresolved. Transplantable HSCs have been verified in the human yolk sac at CS16 (5.5 weeks) before that in the liver (7-8 weeks).17,21 In the placenta, transplantable HSCs were found at week 9 onwards121 and in the second trimester, in another study,17 but HSPCs with fetal HSC phenotype (CD34+CD38loCD90+GPI80+) that expand in HSC cultures were detected at week 5 onwards.20 These discrepancies may be caused by HSC immaturity or technical factors, such as tissue dissociation methods or pregnancy termination using antiprogestins that target placental vasculature. scRNA-seq of CS14 (4.5 weeks) conceptus helped detect similar populations of molecularly defined nascent HSCs in the AGM, placenta, and yolk sac before HSCs appeared in the liver, heart, or head.15 Molecular signatures suggested that extraembryonic HSC–like cells are 1 step away from the most immature AGM HSCs, which exhibit highest expression of endothelial genes and minimal connections to differentiating cells. Surprisingly, extraembryonic HSCs at CS14 are more similar to the first molecularly defined HSCs in the liver 1 or 2 weeks later (CS17) than AGM HSCs in the same CS14 conceptus. Because the liver is directly downstream to the yolk sac and the placenta in the circulatory route, the extraembryonic HSCs are likely on track to colonize the liver.15,61 Despite lacking engraftment ability, CS17 liver HSCs are HOXA-patterned and linked to HOXA+ multilineage progeny, including the fetal erythroid cells, implying that they belong to the HSC lineage. Whether the first HSCs colonizing the liver contribute equally to life-long hematopoiesis than their slightly later counterparts or whether they merely support fetal hematopoiesis is not known. Evidence for gradually increasing HSC potency was found in mouse placenta and livers between E11.5/12.5 and E12.5/13.5, respectively.115 These data further suggest that extensive tendency for proliferation and differentiation in immature HSCs before homeostatic programs are established may negatively correlate with sustained HSC properties.
HSC transition to fetal BM
Onset of human BM hematopoiesis begins with myeloid cell colonization of the cavity of long bones, followed by vascularization at approximately week 8.122 Single-cell and transplantation studies detected long-term engraftable HSCs at approximately week 12,27 and fully active BM hematopoiesis was evident by week 14.27,122 Expression of fetal HSC marker GPI80 was confirmed in the second trimester (15-18 weeks) fetal BM HSCs.20 Fetal BM HSCs have been associated with unique properties, including a differentiation bias to B-cell and granulocytic lineages. Direct comparison of human FL and BM HSC/MPP populations between 17 and 22 weeks using scRNA-seq suggested a shift to a more quiescent state upon transition to fetal BM,25 although this was based on HSC/MPP population as a whole rather than molecularly defined HSCs. Future studies are needed to define how the niche changes between human FL and BM to support HSC maturation and life-long maintenance.
Distinct temporal contributions of HSC-independent progenitors and HSCs to developmental hematopoiesis in mice and humans
Lineage-tracing studies in mice have proven instrumental for dissecting the contributions of various developmental populations to pre- and postnatal hematopoiesis. Flt3-Cre marking helped identify a unique, developmentally restricted liver HSPC population that is absent in postnatal tissues123 but can acquire multilineage engraftment ability upon transplantation. During normal development, their contribution to adult HSCs is minimal, but they can generate innate-like lymphoid cells. A great extent of mouse postnatal lymphopoiesis was also tracked to Flt3-Cre–marked, embryo-derived MPPs, which were postulated to originate from immature HSC precursors, pre-HSCs.48 In contrast, studies on Hlf- and Evi1-reporter mice implied that long-term HSCs, marked by high Evi1/Mecom expression in Hlf+ cells, only contribute significantly to hematopoiesis after birth.93 Lineage tracing using Lyve1-Cre indicated a shift between progenitor-driven erythropoiesis and HSC-driven erythropoiesis during late mouse gestation, which was based on Lyve1-marking that matched yolk sac progenitors vs adult HSCs.124
Although mouse-human comparisons using similar lineage-tracing techniques are not possible, scRNA-seq trajectories in human developmental hematopoietic tissues indicate much greater contribution of transcriptomically defined HSCs to human developmental hematopoiesis than that in mouse tissues. Human embryonic liver at 6 weeks harbors newly colonized HOXA+ HSC that directly connects to similarly patterned erythroid, megakaryocytic, and myeloid cells.15 By week 8, the differentiation trajectories include HSC-derived EBF1+IL-7R+ B-lymphoid cells, which replace HSC-independent SPINK2+IL-7R+ LMPs in the liver at earlier stage. These studies suggest that HSCs contribute substantially to hematopoiesis throughout human gestation, beginning from late embryonic stages (6-8 weeks).15,22,23,25,26 Although these relationships are difficult to confirm during native human developmental hematopoiesis, new tools have been developed to demonstrate clonal relationships, such as mitochondrial DNA mutations and could potentially be used to track human developmental hematopoiesis.125 Nonetheless, the differences between HSC-dependent vs HSC-independent progenitor contributions to mouse and human developmental and postnatal hematopoiesis are profound and may be explained by the longer human gestation (38 weeks vs 3 weeks in mice) and the earlier birth in mice that corresponds to beginning of fetal period (week 9) of human development (Figure 1). These differences should be considered when using the mouse to model human hematological diseases that develop in utero, such as infant leukemias and trisomy 21–associated preleukemic disorder.59
Unique properties of HSC-forming arterial HE
Emergence of human HSCs from arterial endothelium
The cellular origin of HSCs has been difficult to demonstrate because of the intermixing of populations via circulation. Analysis of heartbeat-deficient Ncx1–/– mouse embryos documented multilineage de novo hematopoiesis in the AGM, placenta, and yolk sac, but assaying in vivo HSC potential was hampered by early lethality.117,126-128 Several in vivo and in vitro live-imaging and lineage-tracing models have since documented direct emergence of hematopoietic cells from endothelial precursors via endothelial-to-hematopoietic transition (EHT).129-133 Nevertheless, the identity and location of the unique type of HE that generates HSCs has been poorly defined.
scRNA-seq studies on mouse embryos first documented a link between nascent HSCs and aortic HE64,68 and were followed by studies on human embryos15,16,134 (Figure 3). HSC-primed HE in the human AGM region could be identified based on the coexpression of endothelial markers (CDH5 and SOX7) and HSPC transcription factors (RUNX1 and MYB) in cells that have not yet induced hematopoietic surface markers (PTPRC/CD45 and SPN/CD43). Decreased expression of arterial marker CXCR4 and induction of CD44 pinpointed the arterial subset undergoing EHT.16 Single-cell analysis of CS14/15 AGM tissues uncovered a direct molecular trajectory from HOXA-patterned arterial endothelial cells (ECs) to nascent AGM HSCs via unique arterial endothelial subset, termed pre-HE, which induced IL-33 and ALDH1A1 expression. The decline of IL-33 and the induction of landmarks for HE (KCNK17) and HSPC (SPINK2) marked the progression of EHT (Table 1; Figure 3).15 Spatial transcriptomics helped confirm IL-33, ALDH1A1, KNCK17, and SPINK2 as indicators of human HSC genesis and confirmed this process in IAHCs on the ventral side of the dorsal aorta.15
Hematopoietic progenitors in the CS14/15 AGM or liver that lack HOXA patterning did not show molecular connection to the aortic endothelium in scRNA-seq analyses at this stage, indicating a different origin.15 Nevertheless, HE could already be detected in human embryos at CS1016 (Figure 3). This early HE associated with embryonic hematopoietic cells that were transcriptomically more similar to HSC-independent progenitors in the CS11 yolk sac than to nascent HSCs in the CS13/15 AGM.15 Although both intra and extraembryonic early (CS10-11) hematopoietic cells shared many HSC-associated genes (HLF and SPINK2) and linked to RUNX1+KCNK17+ HE, they expressed unique embryonic genes (LIN28A) and lacked HSC hallmarks (HOXA patterning and HSC stemness regulators MLLT3 and MECOM). In addition, CS10 embryonic HE did not evidence strong arterial identity or hallmarks of pre-HE (IL-33 and ALDH1A1). The HSC-forming HE in CS14/15 AGM was pinpointed as the crossroads between essential arterial endothelial and hematopoietic signaling networks. Although Notch, Wnt, and transforming growth factor β pathways are highly active in arterial EC and pre-HE, attenuation of these pathways at the HE stage implies that their timely suppression may be necessary for HSC emergence.15
These single-cell level molecular observations revealed that although HE generation and EHT can occur in multiple stages and anatomical sites, the outcome of EHT depends on regional patterning (eg, HOXA gene expression characteristic for the AGM region) and the signaling environment where the HE was specified. Activation of hemogenic program in EC without arterial identity generates HSC-independent progenitors. Shifts among these hemogenic programs in the embryo begin at approximately CS11, as the first signs of pre-HE marker IL-33 transcript appear. HSC-forming hemogenic window in the aorta was defined to occur from CS13 to 15,15 after which no molecular connection between AGM arterial endothelium and HSCs could be established,134 implying tight temporal control. Nevertheless, microdissection of CS16 AGM followed by transcriptomics identified a secreted niche factor endothelin 1 (EDN1), expressed ventrally in the aorta near IAHC, which enhances both mouse and human hematopoiesis.134
These studies illustrate the power of single-cell and spatial technologies in pinpointing the cellular origin and intermediates generating human HSCs and defining the environment required for HSC genesis and the timing for signaling switches that occur during EHT. The findings in human embryos concur with studies in mice, in which clonal assays and single-cell transcriptomics concluded that HSCs emerge from Cxcr4+ arterially specified HE, whereas most blood and immune progenitors are derived from Cxcr4− HE.66 To verify clonal relationships between endothelial populations in human embryos and transplantable HSCs using functional assays, it will be important to optimize culture conditions for human HE and nascent HSCs to promote the acquisition of complete HSC potential.
Recapitulating human HSC and progenitor waves from PSCs
Modeling of human developmental hematopoietic waves in vitro
Directed differentiation of human embryonic stem cells and human induced PSCs to HSCs has been improved over the years using many different strategies.3-5 Small molecules and unique PSC reporter lines for hematopoietic regulators have helped in recapitulating and monitoring the early steps of human developmental hematopoiesis that direct mesoderm to distinct hematopoietic waves (Table 2; Figure 4).4 However, generation of long-term reconstituting human HSCs that can function in xenotransplantation has not been achieved solely by directed differentiation but requires viral transduction of HSC regulators and is still very inefficient.77 Nevertheless, transcription factor–driven lineage reprogramming provides a proof-of-concept for HSC generation in vitro.4 Because comprehensive reviews for both approaches are available,3-5,135,136 we focus on novel insights that scRNA-seq studies have provided toward producing HSCs from human PSCs.
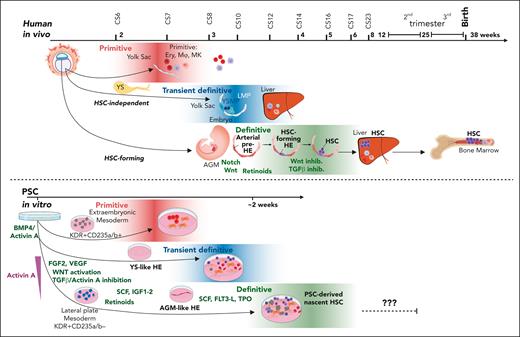
Comparative view of human developmental hematopoiesis in human embryo in vivo and during PSC differentiation in vitro. Top schematic diagram represents developmental hematopoietic waves in humans. The primitive wave composed of primitive erythroid, megakaryocytic, and macrophage progenitors is followed by a transient definitive wave consisting of multiple hematopoietic progenitors (YSPMs and LMPs; Figure 1). These progenitor waves also give rise to long-lasting tissue-resident macrophages and unique γ/δ T-cells. At CS14/CS16, the definitive hematopoietic wave in the embryo gives rise to HSC-forming HE and the HSC lineage. An arterially specified EC in the aorta, characterized by active Notch, WNT, and transforming growth factor βsignaling and patterned by retinoids, undergoes EHT by inhibiting WNT and transforming growth factor β pathways and activating hematopoietic signaling. HSC functional maturation is attained in the liver during following weeks via yet unknown niche signals. It has been possible to recapitulate the early steps of the distinct hematopoietic waves to differentiate PSCs to both YS-like and AGM-like HE precursors and their progeny (bottom). BMP4-mediated mesoderm induction with sequential addition of other morphogens and cytokines, including small molecule–mediated WNT activation, activin A inhibition, and use of retinoids, have been optimized for the generation of diverse intra and extraembryonic-type mesodermal precursors. However, the generation of functionally mature and robustly engraftable human HSCs in culture requires further optimization of the culture microenvironment. SCF, stem cell factor; TPO, thrombopoeitin; VEGF, vascular endothelial growth factor.
Comparative view of human developmental hematopoiesis in human embryo in vivo and during PSC differentiation in vitro. Top schematic diagram represents developmental hematopoietic waves in humans. The primitive wave composed of primitive erythroid, megakaryocytic, and macrophage progenitors is followed by a transient definitive wave consisting of multiple hematopoietic progenitors (YSPMs and LMPs; Figure 1). These progenitor waves also give rise to long-lasting tissue-resident macrophages and unique γ/δ T-cells. At CS14/CS16, the definitive hematopoietic wave in the embryo gives rise to HSC-forming HE and the HSC lineage. An arterially specified EC in the aorta, characterized by active Notch, WNT, and transforming growth factor βsignaling and patterned by retinoids, undergoes EHT by inhibiting WNT and transforming growth factor β pathways and activating hematopoietic signaling. HSC functional maturation is attained in the liver during following weeks via yet unknown niche signals. It has been possible to recapitulate the early steps of the distinct hematopoietic waves to differentiate PSCs to both YS-like and AGM-like HE precursors and their progeny (bottom). BMP4-mediated mesoderm induction with sequential addition of other morphogens and cytokines, including small molecule–mediated WNT activation, activin A inhibition, and use of retinoids, have been optimized for the generation of diverse intra and extraembryonic-type mesodermal precursors. However, the generation of functionally mature and robustly engraftable human HSCs in culture requires further optimization of the culture microenvironment. SCF, stem cell factor; TPO, thrombopoeitin; VEGF, vascular endothelial growth factor.
Major progress toward this goal was achieved using morphogens, including activin A, BMP4, and FGF2, and small molecule agonists and antagonists to direct PSC differentiation to distinct embryonic mesodermal precursors that generate AGM- vs YS-type HE (reviewed previously4) (Figure 4). Stimulation of activin A signaling 1 or 2 days after mesoderm induction generates KDR+CD235a/b+ mesoderm that produces YS-like primitive erythroid cells and macrophages, followed by MPPs that generate γ/δ T-lymphoid cells.137-139 Although multipotent, these YS-like precursors are distinct from the HSC lineage. scRNA-seq analysis helped identify comparable populations in extraembryonic mesoderm of CS7 conceptus.36,137 Inhibition of activin/nodal signaling in the same window promoted the generation of KDR+CD235a/b− mesoderm,139 the precursor for HE that generates AGM-type hematopoietic cells with T-lymphoid potential.73 The expression of medial HOXA genes (HOXA5-9) reflects patterning to definitive, AGM-like hematopoiesis62,63 and is essential for HSC function.25,62 Timed stimulation of retinoic acid (RA) signaling, a key player in AGM-like hematopoiesis in mice,140 could induce HOXA gene expression and other programs critical for HSC formation.62,141 NOTCH activation in PSC-derived HSPC helped specify aorta-like HE that produces multipotent progenitors,142 acting through SOX17-mediated CDX2 induction and orchestration of HOXA genes and arterial programs.74 Despite these advances, transplantable HSCs were not generated in these studies.
Identification of maturation blocks in PSC-derived, AGM-like HSCs using single-cell maps of human developmental hematopoiesis
Single-cell transcriptomics has facilitated the direct comparison of in vitro generated HE and hematopoietic cells to in vivo counterparts from distinct developmental tissues and stages. Comprehensive single-cell maps of human developmental hematopoiesis and immune cell development spanning the early hematopoietic waves in the yolk sac and embryo proper through fetal and neonatal stages15,23,97 are now available and can be used to determine developmental milestones and roadblocks in differentiating PSC to transplantable HSCs or to specific immune cells for cancer immunotherapies. Single-cell analysis of HE and HSPCs generated by combining early WNT stimulation with retinoid agonists revealed that dependance on these pathways distinguishes different mesodermal and HE populations. Although Wnt-dependence categorized intraembryonic mesoderm from extraembryonic mesoderm, RA-dependence subdivided the intraembryonic HE into 2 distinct subsets. RA-dependent HE resembled the HSC-forming HE in CS13 human embryos the most.97,141 Use of artificial intelligence trained using 7- or 17-week old liver HSC/MPPs22 identified a small, transient population of FL-HSC–like cells among human PSC–derived hematopoietic progeny.143 This also revealed higher NOTCH signaling and lower mitochondrial oxidative phosphorylation in in vivo HSPCs, providing evidence that the direct comparison via single-cell transcriptomics can help pinpoint limitations of current PSC differentiation protocols. Neural network–based label transfer method helped compare PSC-HSPCs using in vivo reference map spanning human developmental hematopoiesis and validate PSC differentiation of human induced PSCs to AGM- and placental-like nascent HSCs found in CS14/15 conceptus.15 However, the PSC-derived HSCs were unable to mature to fetal liver HSC stages,15 uncovering a distinct developmental block in differentiation. Stage-specific scorecards illustrating human HSC developmental stages further validated this conclusion.15 A similar approach was used in a PSC differentiation study that used DLL4 and VCAM1 to stimulate NOTCH signaling during EHT, followed by successful T-cell differentiation. Although comparison of the in vitro generated HSPCs to fetal liver scRNA-seq data suggested similarity to FL HSCs, comparison with the scRNA-seq map that also includes early human hemogenic tissues helped verify successful specification to nascent AGM-like HSCs but showed little evidence of maturation to liver stages.144
Lack of HSC developmental maturation of PSC-HSPCs to liver stages is not surprising because most protocols have focused on specification of AGM-like HE. Future efforts will be needed to dissect the signals for HSC functional maturation in FL and BM niches and apply these findings in culture. As the failure of PSC-derived tissue stem cells to mature beyond embryonic stages is observed also with other systems,145,146 overcoming these developmental blocks is a major unsolved problem. Because maturation of human HSCs may require prolonged culture, which in itself is detrimental for human HSCs,147 recent advances in human HSC maintenance or expansion involving unique small molecules (SR1 and UM171),148,149 chemical agonists substituting cytokines,150 or distinct niche cells151 and materials152,153 may help improve the HSC maturation niche. More permissive in vivo models for functional validation of immature human HSC will also be needed. Ability to direct human developmental hematopoiesis in vitro is crucial for establishing disease-in-a-dish-models for inherited diseases, such as hemoglobinopathies or infant leukemias. The new single-cell technologies that can evaluate epigenetic changes, splice isoforms, surface protein expression, and spatial location will provide a lens through which these important developmental events can be studied in humans.
Acknowledgments
The authors thank Sandra Capellera-Garcia for contributing parts of the schematic diagrams, created with BioRender.com.
This work was supported by Sir Henry Dale fellowship 221978/Z/20/Z (Wellcome and Royal Society) (V.C.); Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research at UCLA Interim Research Award and Innovation awards; a Jonsson Cancer Center Foundation and UCLA David Geffen School of Medicine Regenerative Medicine Theme Award; National Institutes of Health (NIH), National Institute of Diabetes and Digestive and Kidney Diseases grants 1RO1DK125097-01, 1R01DK121557-01, and 5R01DK100959-07 (H.K.A.M.); and NIH, National Heart, Lung, and Blood Institute grant 1R01HL162408-01 (H.K.A.M.).
Authorship
Contribution: V.C. and H.K.A.M. wrote the review and designed the illustrations.
Conflict-of-interest disclosure: H.K.A.M. is a member of the scientific advisory board of Notch Therapeutics and a consultant for GraphiteBio. V.C. declares no competing financial interests.
Correspondence: Vincenzo Calvanese, Laboratory for Molecular Cell Biology, University College London, Gower St, London, United Kingdom; e-mail: v.calvanese@ucl.ac.uk; and Hanna K. A. Mikkola, Department of Molecular, Cell and Developmental Biology, University of California Los Angeles, Los Angeles, CA; e-mail: hmikkola@mcdb.ucla.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal