In this issue of Blood, Miller et al examine the role of increased activity of protein phosphatase Mg2+/Mn2+ dependent 1D (PPM1D), also known as wild-type induced phosphatase 1 (WIP1), on chemotherapeutic resistance of hematopoietic stem cells (HSCs).1 Chemotherapeutic resistance of the malignant clones is a common characteristic and is a serious hurdle to our efforts to cure patients with leukemia.2 Elucidating the mechanisms by which leukemic cells evade chemotherapy and/or immunotherapy is important in developing strategies to prevent resistance.
PPM1D is a serine-threonine phosphatase that negatively regulates TP53 and the DNA damage response pathway, as well as other pathways including NF-κB, ATM, and PI3K/AKT.3-6 Truncating mutations of PPM1D in patients with acute myeloid leukemia (AML), myelodysplastic syndrome (MDS), and myeloproliferative neoplasm lead to loss of the C-terminal degradation domain, resulting in increased levels of PPM1D (see figure).7,8 These mutations have been shown to drive clonal hematopoiesis in the presence of certain chemotherapeutics, and approximately 20% of therapy-related MDS or AML harbor a PPM1D mutation.7 In addition, PPM1D overexpression without a PPM1D mutation is common in many cancer subtypes, including leukemia, making PPM1D an enticing therapeutic target.9
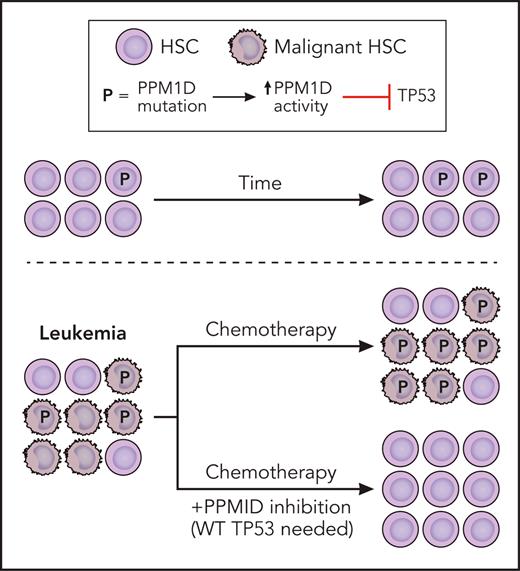
Truncating mutations of PPM1D found in myeloid malignancies lead to loss of the C-terminal degradation domain, resulting in increased levels of PPM1D. Increased PPM1D activity results in inhibition of TP53 that enhances the fitness of both normal and malignant HSCs. Leukemia HSCs that have high PPM1D activity are resistant to chemotherapy. Although PPM1D inhibition alone does not have significant activity against leukemia cells, it is synergistic with chemotherapy. Professional illustration by Patrick Lane, ScEYEnce Studios.
Truncating mutations of PPM1D found in myeloid malignancies lead to loss of the C-terminal degradation domain, resulting in increased levels of PPM1D. Increased PPM1D activity results in inhibition of TP53 that enhances the fitness of both normal and malignant HSCs. Leukemia HSCs that have high PPM1D activity are resistant to chemotherapy. Although PPM1D inhibition alone does not have significant activity against leukemia cells, it is synergistic with chemotherapy. Professional illustration by Patrick Lane, ScEYEnce Studios.
Miller et al, as well as other groups, have previously shown that PPM1D mutations in hematopoietic cells induce resistance to apoptosis and chemotherapeutic agents.7,10 In this issue, Miller et al provide further evidence that PPM1D is a crucial regulator of HSC fitness and that its absence leads to greater sensitivity of malignant cells to chemotherapeutic agents. They developed conditional mouse models of PPM1D truncation (gain of PPM1D) and deletion and used them to evaluate the effects of PPM1D gain and loss, respectively, on the fitness of normal and malignant HSCs. Competitive transplant assays revealed that in normal murine HSCs, PPM1D gain conferred a competitive advantage that was increased in the presence of cytotoxic stress (cisplatin or radiation), whereas PPM1D loss elicited a competitive disadvantage (see figure). They found similar results when they assayed mouse leukemia cells with the fusion MLL-AF9 and either PPM1D gain or loss: the fitness of the leukemia HSC was heightened with PPM1D gain and reduced with PPM1D loss.
The group went on to show through an elegant series of experiments that PPM1D could serve as a therapeutic target in myeloid malignancies. PPM1D loss or treatment with PPM1D inhibitor sensitized murine leukemic HSCs to chemotherapy (see figure). In vitro, PPM1D inhibition was found to be synergistic with a broad array of commonly used chemotherapeutics, including daunorubicin, cytarabine, decitabine, platinum salts, topoisomerase inhibitors, and radiation. The sensitization of daunorubicin and cytarabine with PPM1D inhibition was corroborated in leukemia cells from xenograft models derived from patients with AML. In vivo, MLL-AF9 leukemia xenografts had significantly increased survival when a PPM1D inhibitor was administered in conjunction with doxorubicin and cytarabine.
The actions of PPM1D inhibition are hypothesized to involve multiple pathways due to the numerous known substrates of this phosphatase. The authors, however, clearly demonstrate that TP53 is crucial for the sensitizing of malignant cells to PPM1D inhibition. When compared with knockdown of other modulators of the DNA damage response pathway, inflammation, and the p38 pathway, loss of TP53 instigated the greatest resistance.
With PPM1D mutations and amplifications present across myeloid malignancies, the clinical implications of these findings have the potential to be far reaching. In addition, the authors begin to address the applicability of this approach to solid malignancies. Using a cell line screen, they show that the majority of TP53 wild-type solid tumor cell lines exemplified enhanced sensitivity to daunorubicin with the addition of a PPM1D inhibitor.
It is important to note, however, that PPM1D inhibition on its own did not have significant activity against leukemia cells but rather worked in synergy with chemotherapeutics or radiation (see figure). The question therefore arises as to which therapies would be optimal to combine with PPM1D inhibition. The authors make a compelling argument for DNA-damaging treatments such as chemotherapeutics and radiation, but could this extend to other approaches? Given that PPM1D modulates the immune system, could it serve as a sensitizer for immunotherapeutics? As PPM1D interacts directly with MDM2, could it synergize with MDM2 inhibitors?
Important work lies ahead to translate the compelling findings presented by Miller et al. Although it is reassuring that PPM1D loss did not cause significant hematologic or nonhematologic toxicity in mice, further testing in primary cells will be crucial to understand the specificity of PPM1D inhibition to malignant vs normal HSCs, ensuring that a potential therapeutic window exists. The authors’ finding that PPM1D expression level was associated with response and corroboration of PPM1D as a biomarker for sensitivity to PPM1D inhibition could allow us to determine which patients would derive most benefit from this sensitizer. Furthermore, a greater understanding of the downstream events of PPM1D inhibition will enable us to optimally choose the best combinatorial partners for PPM1D inhibition. On a final note, there is lack of a clinical-grade PPM1D inhibitor, which prohibits clinical translation of these important findings. It is therefore essential that novel PPM1D-targeting agents are developed so the potential of this approach can be further explored.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal