Key Points
Frequent plateletpheresis is associated with lymphopenia, sometimes severe but a robust immune response to vaccine neoantigens is maintained.
Frequent donations and lower total lymphocyte or lower CD4 counts at baseline were not associated with lower vaccine responses.
Abstract
Frequent plateletpheresis is associated with severe lymphopenia of uncertain clinical significance. We assessed the functional impact of frequent platelet donations and associated lymphopenia on the response to neoantigens. We conducted a prospective study of 102 platelet donors (HIV uninfected) who were naive to meningococcal vaccination recruited at Brigham and Women’s Hospital. One dose of quadrivalent meningococcal conjugate vaccine was administered. Seroresponse was defined as a fourfold increase of serum bactericidal antibody titers and seroprotection was defined as postvaccination titers of ≥1:8, for each of the 4 vaccine antigens (A, C, W, and Y). Mean age of participants was 61 years, 69% were male, and medial number of platelet donations in prior year was 14 (interquartile range, 4-20). Frequent platelet donors had a low CD4 count (14% with ≤200/μL and 34% with ≤350/μL). Seroresponse rates varied from 68% for serogroup Y to 86% for serogroup A and were higher for participants with baseline titers of <1:8. Postvaccination seroprotection rates varied from 76% for serogroup Y to 96% for serogroup A. After adjustments for age, sex, and frequent donations, lower total lymphocyte or lower CD4 counts were not associated with lower responses. These data suggest no impairment by plateletpheresis-associated lymphopenia on response to these neoantigens. This trial was registered at www.clinicaltrials.gov as #NCT04224311.
Introduction
Plateletpheresis procedures are frequently performed at blood donor centers. These procedures harvest platelets from volunteer donors, which can then be transfused into patients with severe thrombocytopenia or platelet dysfunction. An important step of plateletpheresis, leukoreduction, is the removal of white blood cells from the platelet unit to prevent alloimmunization and febrile reactions during transfusion.1,2 Commonly, a leukoreduction chamber system (LCS) is used to sequester the white blood cells, which are not retransfused to the donor.
Until recently, there has been conflicting evidence regarding lymphocyte depletion risk in frequent platelet donors. The 1988 US Food and Drug Administration (FDA) guidance suggested limiting the number of collections to 24 from a single donor in 1 year and required that platelet donors give informed consent regarding the unclear long-term effect of plateletpheresis on the reduction of lymphocyte count.3 Given the lack of evidence supporting clinically significant plateletpheresis-associated lymphopenia with modern plateletpheresis instruments, the FDA removed this latter recommendation in its 2007 guidance.4
Recently, Gansner et al found a strong association between frequent plateletpheresis, using the Trima Accel Automated Blood Collection System (Terumo, BCT, Lakewood, CO) that uses the leukoreduction method previously described, and CD4+ T-cell lymphocyte depletion in healthy donors.5 For example, of those donors who had 20 to 24 plateletpheresis procedures in the previous 365 days, 30% had a CD4+ a lymphocyte count of <200 cells per microliter, the threshold for AIDS in HIV-positive individuals. Although lymphopenia did not appear clinically harmful as assessed by a standard donor history questionnaire, it remains unclear whether frequent plateletpheresis is associated with immune dysfunction.
Our study aims to assess the functional impact of frequent platelet donations by evaluating the impact of number of platelet donations on the response to neoantigens, which was assessed by using the quadrivalent conjugate meningococcal vaccine. This vaccine was chosen because conjugate vaccines induce a T-cell dependent immune response, which provides insight into T-cell function6 and because most adults have not been immunized against meningococcal disease.
Methods
Study design
This study was an open-label prospective study evaluating the immunogenicity of a single dose of MenACWY-D (Menactra, Sanofi Pasteur Inc, Swiftwater, PA) in plateletpheresis donors at the Brigham and Women’s Hospital. The research protocol was approved by the Partners Healthcare Institutional Review Board. Donors were recruited from the Brigham and Women’s Hospital and Dana-Farber Cancer Institute blood donation center (Kraft Family Blood Donor Center), from March 2020 to October 2021, and all participants provided written informed consent. Plateletpheresis was performed in accordance with standard protocol using the Trima Accel Automated Blood Collection System (Terumo, BCT, Lakewood, CO). The number of previous successful and completed plateletpheresis procedures were counted for each participant.
At baseline, blood was collected for a complete blood count, CD4 count, and baseline immunogenicity assessment. MenACWY-D was administered thereafter, on the same day, as a 0.5 mL dose via intramuscular injection in the deltoid muscle. If the participant had a plateletpheresis planned on that same day, the blood samples were collected before the plateletpheresis procedure, and the vaccine was given thereafter. Participants were followed up 1 month after vaccination for immunogenicity assessment. They were not excluded from donating between vaccination and immunogenicity assessment, as per standard practices after receipt of a nonlive vaccine.
Study population
Adults who met the standard requirements to donate platelets, and who successfully completed at least 1 plateletpheresis donation in the prior 365 days, were eligible. Volunteers were excluded if they donated platelets at another site in the previous 365 days, had a severe allergy to any component of MenACWY-D vaccine, had a history of a previous meningococcal vaccination or infection, had a history of Guillain-Barré syndrome, or had a current pregnancy or failure to meet contraceptive requirement if of childbearing potential. Before each plateletpheresis donation, the donor’s medical history was reviewed as per routine and blood samples were obtained for FDA-required donor infectious disease screening tests (HIV-1/2, hepatitis B virus, hepatitis C virus, West Nile virus, human T-lymphotropic virus-I/II, Treponema pallidum, and Babesia microti). Platelet donors with immunosuppressive conditions or on immunosuppressive drugs are deferred from donating. In the rare event that a platelet donor has a positive screening test result, the donated unit is discarded, the donor is deferred from donating as per FDA regulations and is also excluded from this study.
Laboratory assessment
To assess the vaccine response, pre- and postvaccination (at 1 month) rabbit serum bactericidal antibody (rSBA) titers against capsular polysaccharide of group A, C, W, and Y meningococcal control strains were measured, as previously described.7 The assay uses baby rabbit serum as an exogenous complement source. Patient sera were kept frozen at –80°C until testing was performed at the Vaccine Evaluation Unit of Public Health England, Manchester, United Kingdom. Laboratory personnel were blinded to participants’ characteristics. Seroresponse was defined as a fourfold increase of rSBA titers between pre- and postvaccination, and seroprotection was defined by postvaccination titers ≥1:8, as previously established as a serological correlate of protection for serogroup C.6,8
Statistical analysis
Analyses included descriptive and graphical summaries. Fisher exact tests were used to compare categorical characteristics. The associations between seroresponse or postvaccination seroprotection and the number of platelet donations in the previous 365 days per CD4 count at vaccination were evaluated using age- and sex-adjusted multivariable logistic regression (refer to supplemental Data for more details). Two-sided P values <.05 were considered statistically significant. All analyses were conducted in R (https://www.R-project.org/).
Results
A total of 102 plateletpheresis donors who not infected with HIV, consented to participate in the study and all were vaccinated with MenACWY-D. Baseline blood was missing for 1 participant, and a 1-month visit blood sample was missing for 1 participant; 100 and 101 participants were therefore included in the seroresponse and the postvaccination seroprotection analysis, respectively. Demographic information and baseline lymphocyte and CD4 counts are shown in Table 1. The median age of participants was 61 years (interquartile range [IQR], 52-67), and 69% were male. The number of successful plateletpheresis donations within 365 days of vaccination ranged from 1 to 24, with a median of 14. Our prospective statistical plan was to dichotomize donations as high vs low for comparison; however, the distribution of donations was graded over this range. Therefore, the number of donations was tested as a quantitative predictor variable rather than a binary variable. On the day of vaccination, the median total lymphocyte and CD4 counts were 1.11 × 103/μL (IQR, 0.76-1.48) and 502 per μL (IQR, 249-662), respectively. There were 14 (14%) and 35 (34%) participants who had a CD4 count of ≤200/μL and ≤350/μL, respectively. Notably, among those who had at least 20 donations in the previous 365 days, 30% (9 of 30) and 77% (23 of 30) had a CD4 count of ≤200/μL and ≤350/μL, respectively. There was an inverse correlation between the number of plateletpheresis in the previous year and the total lymphocyte and CD4 counts (supplemental Figure 1 and 2).
Demographic information and baseline lymphocyte counts (N = 102)
| Median age, y (IQR; range) . | 61 (52-67; 26-89) . |
|---|---|
| Sex | |
| Male, n (%) | 70 (69) |
| Race | |
| White, n (%) | 98 (96) |
| Asian and/or Pacific Islander, n (%) | 3 (3) |
| Black and/or African-American, n (%) | 1 (1) |
| Ethnicity | |
| Non-Hispanic, n (%) | 102 (100) |
| Median number of donations in the last 365 d, n (IQR; range) | 14 (4-20; 1-24) |
| Median number of donations in life, n (IQR; range) | 54 (10-167; 1-551) |
| Median lymphocyte count at vaccination, × 103/μL (IQR; range) | 1.11 (0.76-1.48; 0.36-2.56) |
| Median CD4 count at vaccination, × cells/μL (IQR; range) | 502 (249-662; 92-1289) |
| Median age, y (IQR; range) . | 61 (52-67; 26-89) . |
|---|---|
| Sex | |
| Male, n (%) | 70 (69) |
| Race | |
| White, n (%) | 98 (96) |
| Asian and/or Pacific Islander, n (%) | 3 (3) |
| Black and/or African-American, n (%) | 1 (1) |
| Ethnicity | |
| Non-Hispanic, n (%) | 102 (100) |
| Median number of donations in the last 365 d, n (IQR; range) | 14 (4-20; 1-24) |
| Median number of donations in life, n (IQR; range) | 54 (10-167; 1-551) |
| Median lymphocyte count at vaccination, × 103/μL (IQR; range) | 1.11 (0.76-1.48; 0.36-2.56) |
| Median CD4 count at vaccination, × cells/μL (IQR; range) | 502 (249-662; 92-1289) |
Vaccine responses in the entire cohort
Seroresponse and postvaccination seroprotection for all 4 antigens are shown in Table 2. Four weeks after vaccination, seroresponse rates varied from 68% for serogroup Y to 86% for serogroup A and were generally higher for participants with baseline titers of <1:8. Despite having no history of previous meningococcal vaccination or infection, 22% to 30% of participants had baseline titers of at least 1:8, depending on the serogroup. After vaccination, seroprotection rates varied from 76% for serogroup Y to 96% for serogroup A. One participant who had protective titers for serogroup W at baseline (1:32) had seroreversion.
Seroresponse and postvaccination seroprotection in the cohort, n (%)
| . | A . | C . | W . | Y . | P value seroresponse rate difference (<1:8 - ≥1:8) across serogroup . |
|---|---|---|---|---|---|
| Seroresponse | 86/100 (86) | 78/100 (78) | 83/100 (83) | 68/100 (68) | 0.21 |
| <1:8 prevaccination | 68/73 (93) | 65/79 (82) | 59/70 (85) | 53/78 (69) | |
| ≥1:8 prevaccination | 18/27 (67) | 13/21 (62) | 24/30 (79) | 12/22 (64) | |
| Postvaccination seroprotection∗ | 97/101 (96) | 87/101 (86) | 89/101 (88) | 77/101 (76) | 0.45 |
| <1:8 prevaccination | 69/73 (95) | 65/79 (82) | 59/70 (85) | 54/78 (69) | |
| ≥1:8 prevaccination | 27/27 (100) | 21/21 (100) | 29/30 (97) | 22/22 (100) |
| . | A . | C . | W . | Y . | P value seroresponse rate difference (<1:8 - ≥1:8) across serogroup . |
|---|---|---|---|---|---|
| Seroresponse | 86/100 (86) | 78/100 (78) | 83/100 (83) | 68/100 (68) | 0.21 |
| <1:8 prevaccination | 68/73 (93) | 65/79 (82) | 59/70 (85) | 53/78 (69) | |
| ≥1:8 prevaccination | 18/27 (67) | 13/21 (62) | 24/30 (79) | 12/22 (64) | |
| Postvaccination seroprotection∗ | 97/101 (96) | 87/101 (86) | 89/101 (88) | 77/101 (76) | 0.45 |
| <1:8 prevaccination | 69/73 (95) | 65/79 (82) | 59/70 (85) | 54/78 (69) | |
| ≥1:8 prevaccination | 27/27 (100) | 21/21 (100) | 29/30 (97) | 22/22 (100) |
Prevaccination serostatus was not available for 1 participant.
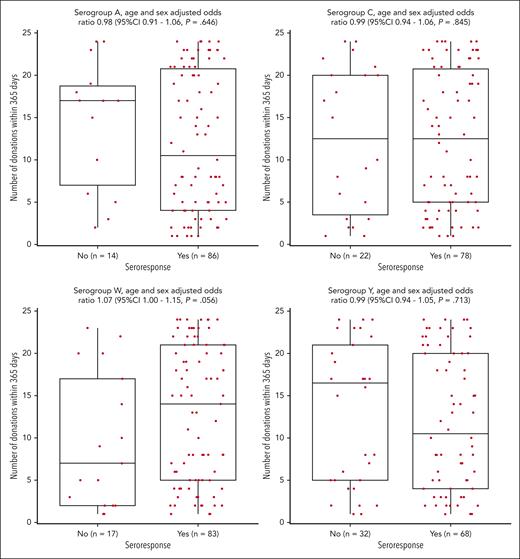
Impact of the number of platelet donation on vaccine responses
After adjustment for age and sex, participants who responded to vaccination had a similar number of successful plateletpheresis procedures in the previous 365 days (Figure 1 and supplemental Figure 3) for all serogroups compared with those who did not respond. Of note, for serogroup W, responders tended to have a higher number of donations in the previous year than those who did not respond. In the subgroup of participants with titers of <1:8 at baseline, the number of previous donations were similar in responders and nonresponders (supplemental Figure 4).
Relationship between seroresponse and the number of platelet donations in the previous 365 days.
Relationship between seroresponse and the number of platelet donations in the previous 365 days.
Impact of the lymphocyte count on vaccine responses
After adjustment for age and sex, participants who seroresponded after vaccination had similar total lymphocyte and CD4 counts at baseline than nonresponders for serogroups A, C, and Y (Figure 2, Figure 3). For serogroup W, responders had significantly lower CD4 counts at vaccination. Similar findings were seen for postvaccination seroprotection (supplemental Figure 5 and 6). In the subgroup of participants with baseline titers of <1:8, total lymphocyte and CD4 count were also similar between responders and nonresponders (supplemental Figure 7 and 8).
Relationship between seroresponse and the total lymphocyte count at vaccination.
Relationship between seroresponse and the total lymphocyte count at vaccination.
There were 35 participants with a CD4 count of ≤350/μL at vaccination. In comparison with those with CD4 count >350/μL at baseline, there was no difference in the rate of seroresponse and postvaccination seroprotection, for all 4 serogroups (Table 3).
Seroresponse and postvaccination seroprotection based on the CD4 count at vaccination
| . | Seroresponse (n = 100) . | Postvaccination seroprotection (n = 101) . | ||||
|---|---|---|---|---|---|---|
| CD4 ≤ 350, n (%) n = 35 . | CD4 > 350, n (%) n = 65 . | P value . | CD4 ≤ 350, n (%) n = 35 . | CD4 > 350, n (%) n = 66 . | P value . | |
| A | 28 (80.0%) | 58 (89.2%) | .23 | 33 (94.3%) | 64 (97.0%) | .61 |
| C | 27 (77.1%) | 51 (78.5%) | 1 | 29 (82.9%) | 58 (87.9%) | .55 |
| W | 29 (82.9%) | 54 (83.1%) | 1 | 31 (88.6%) | 58 (87.9%) | 1 |
| Y | 23 (65.7%) | 45 (69.2%) | .82 | 25 (71.4%) | 52 (78.8%) | .46 |
| . | Seroresponse (n = 100) . | Postvaccination seroprotection (n = 101) . | ||||
|---|---|---|---|---|---|---|
| CD4 ≤ 350, n (%) n = 35 . | CD4 > 350, n (%) n = 65 . | P value . | CD4 ≤ 350, n (%) n = 35 . | CD4 > 350, n (%) n = 66 . | P value . | |
| A | 28 (80.0%) | 58 (89.2%) | .23 | 33 (94.3%) | 64 (97.0%) | .61 |
| C | 27 (77.1%) | 51 (78.5%) | 1 | 29 (82.9%) | 58 (87.9%) | .55 |
| W | 29 (82.9%) | 54 (83.1%) | 1 | 31 (88.6%) | 58 (87.9%) | 1 |
| Y | 23 (65.7%) | 45 (69.2%) | .82 | 25 (71.4%) | 52 (78.8%) | .46 |
Discussion
In this prospective study of 102 platelet donors, we confirm previous observations showing a strong relationship between the frequency of plateletpheresis using the Trima Accel system and lymphopenia.5 However, frequent donations, lower total lymphocyte counts, or lower CD4 counts were not associated with lower responses to neoantigens, suggesting robust functional immunity.
Data are accumulating regarding the association between plateletpheresis and lymphopenia. Instruments using an LCS can lead to severe lymphopenia5,9,10 that may last >1 year after the last donation.11 Non-LCS instruments seem to be associated to a lesser degree with lymphopenia.12 The clinical impact of this lymphopenia is uncertain, but most groups found no association between frequent platelet donations and a higher risk of infection or opportunistic infections.5,9 A recent retrospective cohort study from Sweden found a low absolute rate of infection in platelet donors but a significantly higher risk of infection in more frequent donors.10 Importantly, there were no cases of Pneumocystis jirovecii pneumonia, aspergillosis, disseminated mycobacterial infection, or cryptococcal infection in this study.
In our study, neoantigens (quadrivalent conjugate meningococcal vaccine) were found to be immunogenic in a cohort of platelet donors who had a median of 14 plateletpheresis sessions in the previous 365 days. Vaccine responses were not impaired in frequent donors, despite the profound lymphopenia that we observed with repeated donations. Our findings are consistent with those of a recent study by Laumaea et al, in which similar immune responses to COVID-19 vaccination were observed among a cohort of frequent platelet donors.13
In general, immunocompromised hosts seem to have a lower immune response to the conjugate meningococcal vaccine. In a study of 67 recipients of hematopoietic stem cell transplantation who were vaccinated with Menactra ∼1 year after transplantation, seroresponse rates in nonimmune participants at baseline, using rSBA, were A: 77%, C: 66%, W: 52%, and Y: 65%, and postvaccination seroprotection rates were A: 81%, C : 66%, W: 57%, and Y: 66%.14 In a small study of recipients of solid organ transplantation, postvaccination seroprotection rates after a conjugate meningococcal vaccine (Menveo) were A: 20%, C: 60%, W: 30%, and Y: 40%.15 Another study showed that children, adolescents, and young adults infected with HIV had a significantly lower seroresponse rate (71% vs 100%) compared with controls after the administration of a serogroup-C conjugate vaccine.16 These 3 populations known to have impaired cellular response had a suboptimal response to meningococcal vaccination. This contrasts with the vaccine responses seen in frequent platelet donors in our study, including those with low CD4 count, suggesting there may be no functional impact on response to neoantigens of frequent platelet donation despite objective numerical lymphopenia. However, head-to-head comparison between these studies and ours should be done with caution because the vaccines, the immunogenicity assays, and/or the demographics of participants were different. Of note, for serogroup W, we found that responders had a higher number of donations and lower CD4 counts at vaccination. The significance and the reason of these findings remain unclear.
In the pivotal immunogenicity trial of MenACWY-D described in the package insert, 1280 participants aged 18 to 55 years received a single dose of MenACWY-D.17 Seroresponse rates using rSBA were A: 80.5%, C: 88.5%, W: 89.4%, and Y: 73.5%. In participants who were seronegative at baseline, seroresponse ranged between 91% and 100%. In our study, seroresponse is also higher for those seronegative at baseline, particularly for serogroups A and C, but the overall seroresponse rate seems to be slightly lower than in the pivotal trial. This difference may be explained by age and immunosenescence. In a trial of healthy participants aged ≥56 in the United States, seroresponse of a single dose of MenACWY-D were A: 89%, C: 75%, W:73%, and Y: 54%.18 Baseline seroprotection rates were in 35%, 19%, 33%, and 38%, and postvaccination seroprotection rates were 98%, 79%, 90%, and 71% for serogroups A, C, W, and Y, respectively. These responses seen in healthy individuals are very similar to what was seen in our study.
This study has several limitations. First, this was a single-center study that assessed the impact of frequent plateletpheresis using the Trima Accel system on immunogenicity of a single vaccine. Our study population was also relatively homogenous in terms of demographics, there was no control group who did not donate platelets, and relatively few participants with both very low CD4 count and high platelet donation numbers. Baseline immunoglobulin levels were not routinely measured. The study does not provide information on the risk of infection (although none was observed) but rather the antibody response to vaccination. However, because an rSBA titer of 1:8 is a well-established correlate of protection for serogroup-C meningococcal disease,6,8 this vaccine immunogenicity study provides important information on the quality and the function of the antibodies elicited. It is important to note that 22% to 30% of participants were seropositive at baseline, which is consistent with seroprevalence in individuals who are vaccine naive in the United States18-20 and there were no significant meningococcal outbreak in Massachusetts during the study period.
In conclusion, frequent platelet donation and plateletpheresis-associated lymphopenia are not associated with a lower immune response to neoantigens included in the quadrivalent conjugate meningococcal vaccine. These data are reassuring and suggest that the clinical significance of plateletpheresis-associated lymphopenia is likely limited.
Acknowledgment
This work was supported in part by the Chleck Family Foundation.
Authorship
Contribution: M.D., L.R.B., and R.M.K. designed research; M.D., P.C., X.M., D.P., C.M., T.W., K.O., J.H.K., S.S.V., J.A.K., J.A.G., and J.A.K. performed research; M.D., A.C.S., S.R.W., and L.R.B. analyzed the data; G.Z. and S.P. performed statistical analyses; M.D. wrote the initial manuscript; and all authors read and approved the final manuscript.
Conflict-of-interest disclosure: S.R.W. has conducted clinical trials funded by Janssen Vaccines, ModernaTX, and Sanofi Pasteur. A.C.S. is involved in HIV, coronavirus disease 2019 (COVID-19), and other vaccine clinical trials conducted in collaboration with the National Institutes of Health, HIV Vaccine Trials Network, COVID-19 Vaccine Prevention Network, International AIDS Vaccine Initiative, Crucell/Janssen, and Moderna. L.R.B. is involved in HIV and COVID-19 vaccine clinical trials conducted in collaboration with the National Institutes of Health, HIV Vaccine Trials Network, COVID-19 Vaccine Prevention Network, International AIDS Vaccine Initiative, Crucell/Janssen, Moderna, Military HIV Research Program, the Gates Foundation, and the Ragon Institute. The remaining authors declare no competing financial interests.
Correspondence: Michaël Desjardins, Division of Infectious Diseases, Centre Hospitalier de l’Université de Montréal, 1000 Saint-Denis St, Montréal, QC, Canada H2X 3J4; e-mail: michael.desjardins.med@ssss.gouv.qc.ca.
References
Author notes
Data are available on request from the corresponding author, Michaël Desjardins (michael.desjardins.med@ssss.gouv.qc.ca).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal